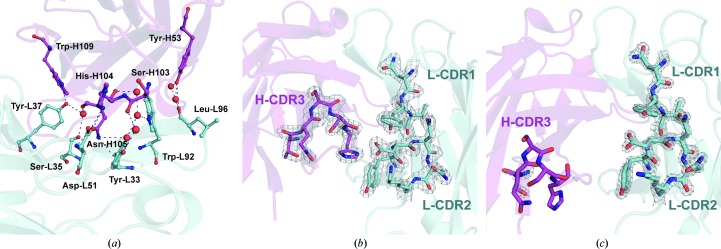
Figure 4.
(a) Interdomain interactions between the CDR loops of A17λ and interactions involving catalytic Tyr-L37. Protein residues involved in these interactions are shown in ball-and-stick representation, water molecules are shown as spheres and hydrogen-bond interactions are shown as blue dashed lines. Here and in (b) and (c), heavy and light chains are shown in magenta and cyan, respectively. Tyr-L33 and Ser-L35 belong to L-CDR1, Asp-L51 to L-CDR2, Trp-L92 and LeuL96 to L-CDR3, Tyr-H53 to H-CDR2, and Ser-H103, His-H104 and Asn-H105 to H-CDR3. (b) 2F o − F c electron-density map contoured at the 2σ level (0.5 e Å−3) above the mean for the CDR loop region of A17λ. (c) 2F o − F c electron-density map contoured at the 2σ level (0.5 e Å−3) above the mean for the CDR loop region of A17κ.