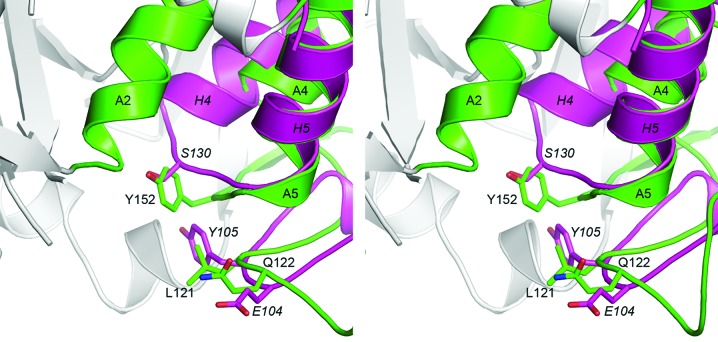
Figure 4.
Stereoview of the superposition of ADC-1 (green) and TEM-1 (magenta). Although the polypeptide between ADC-1 helices A4 and A5 (the P2-loop comprising 40 residues unique to the class C enzymes) is substantially longer and is folded completely differently from the corresponding loop between TEM-1 helices H4 and H5 (which comprises only four residues), two residues at the end of the P2-loop (Leu121 and Gln122) occupy the same locations as the two residues at the end of the longer TEM-1 P-loop (Glu104 and Tyr105). The side chain of residue Tyr152, which is critical in the catalytic mechanism of the class C enzymes, is structurally equivalent to the conserved Ser130 side chain in the class A enzymes.