Figure 2.
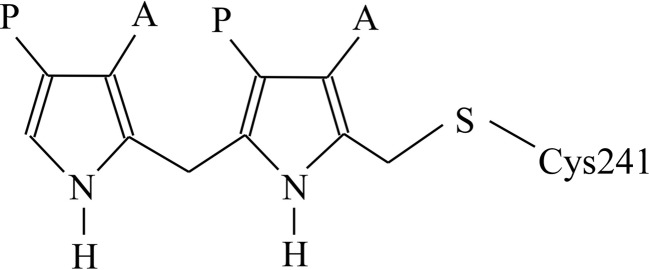
The dipyrromethane cofactor of porphobilinogen deaminase is covalently attached to the enzyme by a thioether bond to a cysteine residue. Four substrate pyrroles are added linearly to the cofactor; finally, hydrolysis of the linkage between the substrate and the cofactor releases the tetrapyrrole product hydroxymethylbilane.
