Abstract
Background
Blockade of platelet activation and aggregation can inhibit metastasis in preclinical models and is associated with cancer prevention. To test whether disruption of platelet function with clopidogrel and aspirin would decrease the number of circulating tumor cells (CTCs) in patients with metastatic breast cancer, a randomized phase II study was performed.
Methods
Patients with metastatic breast cancer who were not currently receiving cytotoxic chemotherapy were eligible. Patients were randomized to receive either clopidogrel and aspirin or to a control group receiving no treatment. Phlebotomy was performed at baseline, at 2 and 4 weeks, and monthly thereafter to obtain specimens to assess CTC, platelet aggregation, and thrombin activity. The primary end point was the proportion of patients with detectable CTCs at 1 month.
Results
Forty-eight patients were enrolled and 42 were evaluable at 1 month. Baseline CTC numbers were ≥ 5 in 13% and ≥ 1 in 65% of patients. Despite adequate platelet function inhibition in the treatment group, the proportion of patients with detectable CTCs was similar between the clopidogrel/aspirin and control groups at baseline (P = .21) and 4 weeks (P = .75), showing no treatment effect. Measured endogenous thrombin potential did not correlate with CTC number. No bleeding-related serious adverse events (SAEs) occurred.
Conclusion
The baseline CTC numbers were lower than expected, decreasing the ability to detect an impact of platelet inhibition on CTCs. Clopidogrel and aspirin were well tolerated. Future studies evaluating the potential therapeutic role of antiplatelet therapy in breast cancer remain of interest, and they may be informed by these results.
Keywords: Breast cancer, Circulating tumor cells, Platelet inhibitors
Introduction
The metastatic process is complex and involves migration from the primary tumor site, vascular invasion, evasion of host immune defenses, dissemination (often as microemboli with tumor cells, platelets, and other blood cells), extravasation, and proliferation. Cancer can be associated with a prothrombotic state, increasing the risk of venous thromboembolic events.1,2 Blood components have been implicated in playing a direct role in the development of metastatic disease.3 Platelets may play a crucial role in the metastatic process by affecting tumor cell arrest and adhesion at distant metastatic sites and can protect tumor cells from immune attack.4 Gasic et al. demonstrated that lowering the platelet count in mice resulted in decreased lung invasion after intravenous injection of tumor cell lines.5 Furthermore, antibodies directed against platelet antigens involved in tumor cell adhesion decreased lung tumors in mice after intravenous administration.6-8 Preclinical models have demonstrated that platelets together with tumor-secreted proteins influence the premetastatic bone microenvironment and may promote the metastatic process.9 In vivo experiments have shown that thrombin, a platelet activator, increases pulmonary metastasis, whereas a thrombin inhibitor (r-hirudin), decreases melanoma pulmonary metastases.6,10-13 Clinically, it has been shown that the addition of antiplatelet therapy to chemoradiotherapy in the treatment of patients with small-cell lung cancer prolongs duration of remission and overall survival.14
Studies that have used single-agent antiplatelet therapy in cancer have been promising. It is of note that in the clinical management of cardiovascular diseases, dual-platelet therapies have shown superior results. Circulating tumor cell (CTC) number has been associated with overall and progression-free survival in women with metastatic breast cancer.15 To investigate the effect of dual-antiplatelet therapy on CTCs in women with metastatic breast cancer, a randomized controlled study was conducted using clopidogrel and aspirin to test the hypothesis that platelet inhibition decreases the number of CTCs, a surrogate for cancer outcomes.
Patients and Methods
Patients and Treatment
Women without actively progressing metastatic breast cancer who were not currently receiving chemotherapy were eligible. Previous chemotherapy (adjuvant or metastatic) was permitted. Concurrent endocrine therapy (for at least 2 months before enrollment), bisphosphonate therapy, and/or trastuzumab were permitted. Patient care was directed by the treating physician as clinically indicated. Eligible patients had an estimated survival ≥ 3 months, no platelet inhibitor therapy within 1 month of study, platelet count ≥ 100,000/mm3, international normalized ratio within 0.81 to 1.20, and normal kidney and liver function by institutional standard laboratory evaluation. Exclusion criteria were planned surgery, serious bleeding disorders, history of significant bleeding related to peptic ulcer disease, standing therapy with nonsteroidal antiinflammatory drugs or other platelet inhibitors, and anticoagulant therapy. The institutional review board approved the trial and written consent was obtained from all patients before enrollment.
Patients were randomized to receive either clopidogrel (300 mg loading dose followed by 75 mg orally daily) and aspirin (325 mg orally daily) combination therapy, or no study treatment. Initially, study therapy could be continued until tumor progression, discontinuation of therapy because of an adverse event (AE), or withdrawal of consent. The protocol was later amended to change the maximum study duration to 6 months.
Measurement of Circulating Tumor Cells
CTCs were measured at baseline, at 2 weeks and 4 weeks, and then monthly thereafter. Patient blood samples were collected into 10-mL CellSave (Veridex, Raritan, NJ) tubes and were stored at room temperature and subsequently processed within 72 to 96 hours of collection. CTCs were isolated and measured using the CellSearch assay (Veridex, Raritan, NJ). Samples were analyzed at Washington University with the exception of those collected on Fridays, which were sent to Quest Diagnostics (Madison, NJ) to avoid a processing delay. CTC numbers are reported per 7.5 mL of blood, and the limit of detection for the CellSearch assay is 1 CTC/7.5 mL of blood. The technical details of CellSearch have previously been described.16
Platelet Function Testing
To show study treatment pharmacologic efficacy, blood samples were collected for platelet function testing using the VerifyNow system (Accumetrics, San Diego, CA) with P2Y12 and aspirin cartridges. VerifyNow is a turbidimetric-based optical detection system that measures aspirin or clopidogrel inhibition of platelet aggregation.17 Citrated whole blood is added to a test cartridge containing fibrinogen-coated beads and a platelet activator: adenosine diphosphate (ADP) with prostaglandin E (to specifically activate the ADP P2Y12 receptor in the case of clopidogrel) or arachidonic acid (to synthesize thromboxane A2 in the case of aspirin). Aggregation of activated platelets to fibrinogen-coated beads increases light transmittance, which is reported in aspirin reaction units (ARUs) or P2Y12 reaction units (PRUs). Lower ARU and PRU results are expected when patients take aspirin and clopidogrel.17 Percent inhibition of platelet function by clopidogrel is determined by dividing ADP-induced platelet aggregation (PRU) by thrombin-induced platelet aggregation.
Given the association of malignancy and hypercoagulability and the possible activating effect of CTCs on platelets, the global hemostatic function was evaluated. Thrombin generation, which measures the overall thrombogenic potential of the plasma sample, was measured in patient plasma samples using the Calibrated Automated Thrombogram system (CAT) (Diagnostica Stago, Parsippany, NJ) and the endogenous thrombin potential percentage (ETP%) was calculated. ETP% is a global measure of the thrombogenic potential, as opposed to the more traditional tests such as prothrombin time and the activated partial thromboplastin time, which measure the activity of the extrinsic pathway of coagulation or the intrinsic pathway, respectively. The CAT system is based on the principles for automated thrombin generation measurement as described previously.18 Briefly, platelet-poor plasma is combined with an activator containing phospholipids and human recombinant tissue factor. Generated thrombin cleaves a peptidyl substrate specific for thrombin, which releases fluorescent 7-amino-4-methylcoumarin. Thrombin-generation curves are plotted as thrombin concentration vs. time, with endogenous thrombin time (ETP) being the area under the curve. ETP% is then generated by normalizing the experimental sample with the ETP from a normal plasma sample.
Statistical Analysis
The primary end point was proportion of patients with detectable CTCs at 1 month. It has been shown previously that more than 50% of patients with metastatic breast cancer have detectable numbers of CTCs in the blood.15 Power analysis determined that 76 patients would need to be enrolled to detect a 30% absolute reduction in patients with detectable CTCs, assuming 50% prevalence of CTCs at baseline (80% power with 2-sided significance level of .05). Statistics were generated using the statistical programs SPSS version 17.0.1 (SPSS, Inc, Chicago, IL) and Microsoft Excel (Microsoft Corp, Redmond, WA). Baseline patient characteristics were compared between groups using the χ2 or Fisher exact test for noncontinuous variables and the 2-sample t test for continuous variables. The proportion of patients with detectable CTCs was compared at baseline, 2 weeks, and 1 month by the Fisher exact test. Platelet function was compared between groups at different time points using the Mann-Whitney test (2-tailed). Mean ETP% was compared between groups using the 2-sample t test. ETP% was also correlated with CTC number (Pearson 2-tailed test).
Results
Patient Characteristics
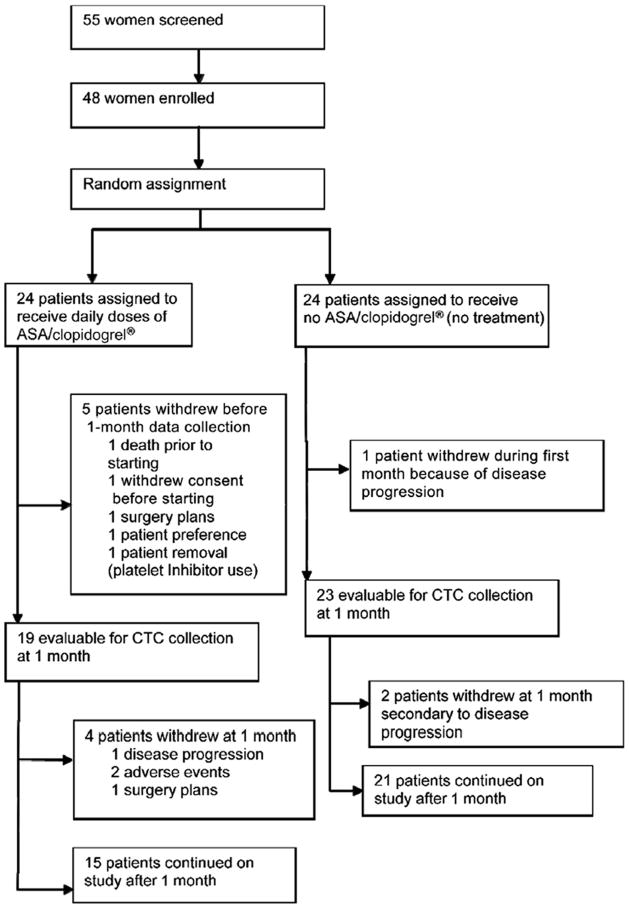
Forty-eight patients were treated in our study, 24 of whom were randomized to treatment and 24 to the control group. The patient flow chart is shown in Figure 1. Two patients in the treatment arm dropped out at or before 1 month because of AEs. One of the AEs was bleeding, which was thought to have resulted from treatment, and the other was back pain deemed to be unlikely to be associated with treatment. Nineteen patients in the treatment arm and 23 patients in the control arm were evaluable for the primary end point.
Figure 1.
The CONSORT Trial Diagram Details the Flow of Patients Through Various Stages of the Trial and the Reason for Patient Withdrawal up to and Just After 1 Month. Five Patients Continued in the Trial Beyond 6 Months; However, Enrollment Time was Later Limited to 6 Months
Patient baseline characteristics are shown in Table 1 and are well balanced overall between groups. More than half of the patients had received 2 or more previous chemotherapy regimens for metastatic disease, and more than 60% of patients had at least 2 sites of metastatic disease. The mean age was 50.7 years in the treatment group and 58.4 years in the control group. Across groups, baseline CTCs were ≥ 5 in only 13% of patients and were ≥ 1 in only 65% of the patients. The low percentage of patients with baseline detectable CTCs prompted discontinuation of the study (because of futility) before all 76 patients were enrolled. Baseline platelet counts and platelet function test results were no different between groups.
Table 1. Baseline Patient Characteristics.
| Characteristic | Control (n = 24) | Treatment (n = 24) | Valid n (Control) | Valid n (Treatment) |
|---|---|---|---|---|
|
| ||||
| Mean age, years | 58.4 | 50.7 | 24 | 24 |
|
| ||||
| Baseline positive CTCs, no. (%) | 18 (75) | 12 (55) | 24 | 22 |
|
| ||||
| Mean baseline platelet count, k/mm3 | 243.7 | 231.6 | 23 | 23 |
|
| ||||
| Mean ARU | 585.4 | 604.8 | 23 | 20 |
|
| ||||
| Mean baseline clopidogrel platelet function, % platelet inhibition | 11.9 | 10.3 | 24 | 22 |
|
| ||||
| HER2+ no. (%) | 10 (42) | 8 (33) | 24 | 24 |
|
| ||||
| ER+, no. (%) | 13 (54) | 15 (62) | 24 | 24 |
|
| ||||
| Number of metastatic sites, no. (%) | – | – | 24 | 24 |
|
| ||||
| 1 | 9 (38) | 8 (33) | – | – |
|
| ||||
| 2 | 7 (29) | 7 (29) | ||
|
| ||||
| ≥ 3 | 8 (33) | 9 (38) | ||
|
| ||||
| Number of previous metastatic chemotherapy regimens, no. (%) | – | – | 24 | 24 |
|
| ||||
| 0 | 4 (17) | 6 (25) | – | – |
|
| ||||
| 1 | 8 (33) | 5 (21) | – | – |
|
| ||||
| 2 | 6 (25) | 4 (17) | – | – |
|
| ||||
| ≥ 3 | 6 (25) | 9 (37) | – | – |
|
| ||||
| Number of previous metastatic endocrine therapies, no. (%) | – | – | 24 | 24 |
|
| ||||
| 0 | 11 (46) | 8 (33) | – | – |
|
| ||||
| 1 | 3 (12) | 7 (29) | – | – |
|
| ||||
| ≥ 2 | 10 (42) | 9 (38) | – | – |
|
| ||||
| Concurrent trastuzumab use, no. (%) | 9 (38) | 5 (21) | 24 | 24 |
|
| ||||
| Concurrent bisphosphonate use, no. (%) | 9 (38) | 12 (50) | 24 | 24 |
|
| ||||
| Smoking, no. (%) | 5 (22) | 4 (17) | 23 | 23 |
Abbreviations: ARU = aspirin reaction units; ER = estrogen receptor; HER = human epidermal growth factor.
Circulating Tumor Cell Responses
The proportion of patients with detectable CTCs (≥ 1 CTC) was calculated at baseline, 2 weeks, and one month, with the primary end point being at 1 month (Table 2). One quarter of the patients in the observation arm had no CTCs present at baseline, whereas nearly one half of those receiving the study therapy lacked baseline CTCs. Changes in CTC number from baseline to 1 month were similar between treatment and control groups, with no discernible trends in those with CTC-positive or CTC-negative status. The CTC status remained fairly stable over time (Fig. 2) (6-month data are not shown), with no more than 25% of patients ever having more than 5 CTCs. Of the 4 patients who had early disease progression before or shortly after the first month of study, CTC count increased by 1, 5, and 19 cells for each of 3 patients respectively, and decreased by 1 cell in 1 patient.
Table 2. Proportion of Patients with Detectable CTCs at 0 and 4 Weeks.
| Time of Determination | Treatment Group | Proportion + CTCs | Proportion − CTCs | P Value (2-tailed) |
|---|---|---|---|---|
|
| ||||
| Baseline CTCs | No treatment | 18 of 24 = 75% | 6/24 = 25% | .21 |
|
| ||||
| Treatment | 12 of 22 = 55% | 10/22 = 45% | – | |
|
| ||||
| 4-Wk CTCs | No treatment | 15 of 23 = 65% | 8/23 = 35% | .75 |
|
| ||||
| Treatment | 11 of 19 = 58% | 8/19 = 42% | – | |
Abbreviation: CTCs = circulating tumor cells.
Figure 2.
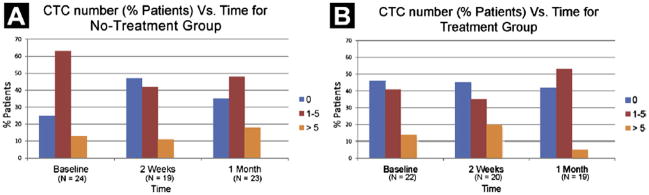
Percentage of Patients With a Given Number of Circulating Tumor Cells (CTCs) (Broken Down by Categories) is Plotted Against Time. N = number of Patients With Valid Data at Each Time Point. Patients Included in 2-Week and 4-Week Measurement Categories had Measurements Performed Between 11 and 21 Days (Median 14 Days) and 21 and 49 Days (Median 28 Days) After Starting Therapy, Respectively. (A) Percent of Patients With a Given Circulating Tumor Cell (CTC) Number is Plotted vs. Time for Patients in the no-Treatment (Control) Group. (B) Percent of Patients With a Given CTC Number is Plotted vs. Time for Patients in the Treatment Group
Platelet Function and Activation Responses
Platelet function response to aspirin and clopidogrel was assessed using specific platelet-function tests for each drug. Suggesting compliance with study therapy, the data indicate that the treatment group experienced a significant inhibition of platelet function at the 2- and 4-week time points for both drugs (P < .001 for each) (Fig. 3). Both clopidogrel- and aspirin-mediated platelet inhibition were similar across all time points for the control group. These results demonstrate pharmacologic treatment effect in the aspirin/clopidogrel group when compared with the control group.
Figure 3.
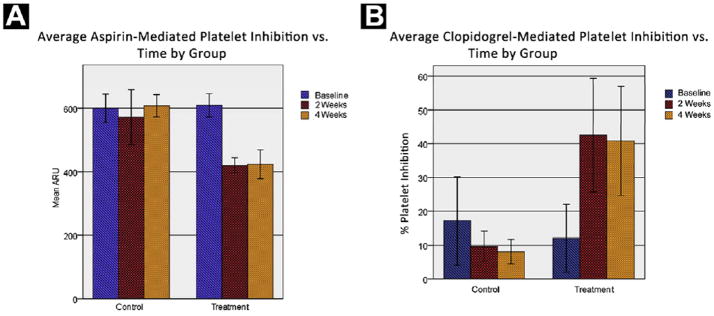
Mean Platelet Inhibition vs. Time is Plotted for Treatment and Control Groups for Baseline, 2-Week, and 4-Week Measurement Time Points for Both Aspirin-Mediated Platelet Inhibition (A) and Clopidogrel-Mediated Platelet Inhibition (B). Patients Included in 2-Week and 4-Week Measurement Categories had Measurements Performed Between 11 and 21 Days (Median 14 Days) and 21 and 49 Days (Median 28 Days), Respectively, After Starting Therapy. Cases Were Excluded if Platelet Function Information was Missing for any Time Point. Percent Platelet Inhibition a (P2Y12 Reaction Units)/(Thrombin Induced Aggregation)
The thrombogenic potential as assessed by ETP% did not correlate with CTC number (Table 3), and the mean ETP% was not different between treatment and control groups (data not shown). ETP% did not correlate with treatment, age, estrogen receptor or HER2 status, or number of metastatic sites (Table 3). These data suggest that this global marker of coagulability potential, the ETP%, was not associated with CTC number or antiplatelet treatment in patients with previously treated metastatic breast cancer.
Table 3. Correlation of CTCs and ETP%.
| Variable | Statistical Analysis | ETP% Baseline | ETP% 4 Weeks |
|---|---|---|---|
|
| |||
| CTC (baseline) | Pearson correlation | −0.163 | −0.339 |
|
| |||
| P value (2-tailed) | 0.480 | 0.156 | |
|
| |||
| n | 21 | 19 | |
|
| |||
| CTC (4 wk) | Pearson correlation | −0.008 | −0.319 |
|
| |||
| P value (2-tailed) | 0.974 | 0.183 | |
|
| |||
| n | 20 | 19 | |
|
| |||
| Treatment group | Pearson correlation | −0.279 | −0.112 |
|
| |||
| P value (2-tailed) | 0.221 | 0.648 | |
|
| |||
| n | 21 | 19 | |
|
| |||
| Age | Pearson correlation | −0.165 | −0.023 |
|
| |||
| P value (2-tailed) | 0.474 | 0.925 | |
|
| |||
| n | 21 | 19 | |
|
| |||
| HER2+ | Pearson correlation | −0.001 | −0.046 |
|
| |||
| P value (2-tailed) | 0.996 | −0.852 | |
|
| |||
| n | 21 | 19 | |
|
| |||
| ER | Pearson correlation | 0.214 | −0.027 |
|
| |||
| P value (2-tailed) | 0.351 | 0.914 | |
|
| |||
| n | 21 | 19 | |
|
| |||
| Metastatic sites | Pearson correlation | −0.214 | −0.079 |
|
| |||
| P value (2-tailed) | 0.353 | −0.746 | |
|
| |||
| n | 21 | 19 | |
Abbreviations: ETP% = endogenous thrombin potential percentage; ER = estrogen receptor; HER = human epidermal growth factor.
Safety
There were no serious AEs (SAEs) related to bleeding or bruising in the treatment group. There were 4 bleeding-related AEs and 15 AEs caused by bruising. The only SAEs that were deemed to be possibly related to treatment were mucositis (grade 3 in 1 patient) and grade 3 nausea, lymphopenia, and edema (all in a second patient). All other SAEs were either deemed unrelated or unlikely to be related to treatment. Although a high incidence of grade I/II bruising was noted, aspirin and clopidogrel were well tolerated in this population of patients with metastatic breast cancer.
Discussion
Preclinical and epidemiologic data suggest that platelet activity may affect progression of breast cancer. CTCs have been shown to correlate with disease outcomes in women with breast cancer.15,19-21 This randomized phase II study of women with metastatic breast cancer treated with dual-antiplatelet therapy or no treatment demonstrated a pharmacologic effect with platelet inhibition that did not correspond with changes in CTCs at 1 month. Similarly, thrombin generation, as measured by ETP%, did not correlate with changes in CTCs at 1 month. There was no significant difference between patients who received antiplatelet therapy and those who did not with respect to the proportion of detectable CTCs at 1 month. Dual-antiplatelet therapy was well tolerated in patients with metastatic breast cancer.
This clinical trial enrolled patients regardless of baseline CTC values. Only 13% of patients had ≥ 5 CTCs at baseline and approximately one-third had no detectable CTCs. These low CTC numbers were much lower than expected and may reflect the study population at large and status of tumor control at point of study entry. The inclusion of patients who had no or few CTCs at baseline limited the ability to distinguish patients who may have benefited from antiplatelet therapy by the calculated 30% absolute reduction in CTCs at 1 month. The study was closed before the planned enrollment of 76 participants because of the low probability of achieving statistical significance with regard to the primary end point; hence, these results cannot not be considered definitive. We performed a theoretical power calculation to determine the number of patients needed if only patients with ≥ 5 CTCs were included in the study. Two hundred eighty patients would need to be enrolled to detect a 10% absolute reduction (66% relative reduction) in patients who have ≥ 5 CTCs, assuming the 15% prevalence of patients with CTCs ≥ 5 at baseline that we actually observed in this study (80% power with 2-sided significance level of .05). Therefore, performing a similar study that mandates higher CTC levels for patient inclusion would require significantly larger enrollment numbers and may not be feasible.
This study was not designed to assess a rise in CTCs; however, the data from the 42 randomized patients who completed 1 month follow-up does not suggest that dual-antiplatelet therapy impedes the rate of CTC increase. The percentage of patients with ≥ 1 CTC remained relatively stable across time and study arm at 1 month. Again, this may reflect the patient population and disease control, as well as the brief duration of the study.
Given the relatively homogeneous and low number of CTCs seen during our study, it is possible that an anticancer response to anti-platelet therapy could have been observed if other methods to measure tumor progression were incorporated into the study design (eg, radiographic measurements, alternative tumor markers, or more sensitive CTC evaluation). A recent report performed a comparison of CTC’s to carcinoembryonic antigen and cancer antigen 15–3 in patients with metastatic breast cancer and found that the biochemical and cellular biomarkers resulted in similar prediction of progression-free survival19. In our study, radiographic assessments were not performed, and would not be expected to be markedly changed in one month’s time. In hindsight, preselecting patients with high CTC numbers may have increased the ability to observe a treatment effect, and the tests for global hemostatic potential such as thrombin generation could be used as a screening tool to select patients.
In the 42 patients who underwent serial assessment, it is unlikely that there would have been genetic variability significant enough to have confounded the results. However, it is of note that in preclinical studies, germline deletion of an important membrane-bound protein involved in platelet aggregation, alphaII(b)beta3 integrin, significantly decreased the burden of bone metastases in mice intravenously inoculated with melanoma cells.22 Likewise, specific pharmacologic inhibition of alphaII(b)beta3 decreased metastases. Antibodies blocking alpha(v)beta3 and alphaII(b)beta3 integrins have produced a reduction in tumor size in mice.23 Additionally, the combined use of an apyrase/ADPase (APT102) and an inhibitor of thromboxane synthesis (aspirin) resulted in significantly decreased breast cancer and melanoma bone metastases in mice.24
Several epidemiologic studies have suggested a chemopreventive effect of aspirin on the development of various cancers, particularly colorectal cancer, as well as cancers of the stomach, breast, and lung.25 However, the results of clinical trials addressing platelet inhibition as primary cancer prevention have been mixed.26-28 The Women’s Health Study, which randomized 39,876 women to receive aspirin therapy or not showed a trend in reduction of lung cancer but no effect for many of the other major cancers.27 Conversely, pooled results from 2 smaller randomized trials showed a significant reduction in the risk of colorectal cancer developing (hazard ratio, 0.74; confidence interval, 0.56-0.97; P = .02).28 In patients with colon cancer, adjuvant aspirin therapy failed to increase relapse-free survival.29 Similarly, in patients with ovarian cancer, a derivative of dipyridamole, RA233, did not show any benefit with respect to disease progression and overall survival when combined with standard treatment.30
The preclinical and epidemiologic studies suggesting anticancer effects of antiplatelet therapy were not refuted by this randomized phase II study. The negative results from this translational randomized phase II study highlight the challenges of biomarker-driven trials and provide data pertinent to designing future studies that may test platelet inhibitors in patients with breast cancer. Future studies testing this hypothesis and the biological characteristics of platelets affecting cancer outcomes remain warranted.
Conclusion
We conducted a randomized phase II study in patients with metastatic breast cancer to test the hypothesis that antiplatelet therapy may decrease the number of CTCs. Despite adequate platelet function inhibition in patients treated with aspirin and clopidogrel, no difference in the proportion of detectable CTCs was noted between the treatment and control groups at 1 month. Overall, aspirin and clopidogrel were relatively well tolerated, with no observed SAEs from bleeding. This study helps to underscore the challenges of biomarker-driven trials and can help inform the design of future studies aimed at assessing the role of antiplatelet therapy in treating breast cancer.
Clinical Practice Points.
Blockade of platelet activation and aggregation has been shown to inhibit metastasis in preclinical models and is associated with cancer prevention in humans.
Currently, the role of antiplatelet therapy in treating and preventing breast cancer remains undefined.
In this study, we demonstrated that antiplatelet therapy did not have an apparent effect on the number of CTCs in patients with metastatic breast cancer.
CTC number was uniformly low in this study population of patients who had relatively stable, heavily pretreated metastatic disease.
Preselecting patients for studies with a high number of CTCs may help to improve the utility of CTCs as a surrogate outcome measure in therapeutic trials.
Future studies investigating the utility of antiplatelet therapy in treating breast cancer are needed.
Acknowledgments
The authors thank Drs David Kipnis, Evan Sadler, John DiPersio, and Paula Fracasso for their valuable suggestions and criticisms. We thank Dr Bill Shannon and Feng Gao for biostatistics expertise and help with analyses. We thank research coordinator Susan Fox (Siteman Cancer Center Developmental Therapeutics Department) for her expert data management. We thank Drs Matthew Ellis and Cynthia Ma for enrolling patients. We thank Rhonda Porche-Sorbet for running the platelet function tests. We also thank Dr Paul Riley from Diagnostica Stago, Inc for running the CAT assay. Most importantly, we thank the study participants for their altruism and dedication to clinical and translational research.
This clinical trial was supported by the Barnes Jewish Foundation, the St. Louis Men’s Group Against Cancer, and the Siteman Cancer Center Developmental Therapeutics program. We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and the Barnes-Jewish Hospital in St. Louis, Missouri, for the use of the Clinical Trials Core services. The Siteman Cancer Center is supported in part by National Cancer Institute Cancer Center Support Grant No. P30 CA91842. KNW and JGS were supported by R01 (CA097250), JGS was supported by a grant from the German Research Foundation (DFG) SCHN682/3-1, RPR and PEL were supported by Washington University School of Medicine. CVP was supported in part by NIDCR K23 DE02019701.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Gouin-Thibault I, Achkar A, Samama MM. The thrombophilic state in cancer patients. Acta Haematol. 2001;106:33–42. doi: 10.1159/000046587. [DOI] [PubMed] [Google Scholar]
- 2.Wun T, White RH. Venous thromboembolism (VTE) in patients with cancer: epidemiology and risk factors. Cancer Invest. 2009;27(suppl 1):63–74. doi: 10.1080/07357900802656681. [DOI] [PubMed] [Google Scholar]
- 3.Kakkar AK, Levine MN. Thrombosis and cancer: implications beyond Trousseau. J Thromb Haemost. 2004;2:1261–2. doi: 10.1111/j.1538-7836.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo JS, Talmage KE, Massari JV, et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133–41. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci U S A. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karpatkin S, Pearlstein E, Ambrogio C, et al. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81:1012–9. doi: 10.1172/JCI113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis JL, Amirkhosravi A. Effect of antihemostatic agents on experimental tumor dissemination. Semin Thromb Hemost. 2002;28:29–38. doi: 10.1055/s-2002-20562. [DOI] [PubMed] [Google Scholar]
- 8.Pearlstein E, Ambrogio C, Karpatkin S. Effect of antiplatelet antibody on the development of pulmonary metastases following injection of CT26 colon adenocarcinoma, Lewis lung carcinoma, and B16 amelanotic melanoma tumor cells into mice. Cancer Res. 1984;44:3884–7. [PubMed] [Google Scholar]
- 9.Kerr BA, McCabe NP, Feng W, et al. Platelets govern pre-metastatic tumor communication to bone. Oncogene. 2013;32:4319–24. doi: 10.1038/onc.2012.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu L, Lee M, Campbell W, et al. Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood. 2004;104:2746–51. doi: 10.1182/blood-2004-03-1047. [DOI] [PubMed] [Google Scholar]
- 11.Rickles FR, Patierno S, Fernandez PM. Tissue factor, thrombin, and cancer. Chest. 2003;124(3 suppl):58S–68S. doi: 10.1378/chest.124.3_suppl.58s. [DOI] [PubMed] [Google Scholar]
- 12.Nierodzik ML, Plotkin A, Kajumo F, et al. Thrombin stimulates tumor-platelet adhesion in vitro and metastasis in vivo. J Clin Invest. 1991;87:229–36. doi: 10.1172/JCI114976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karpatkin S. Role of thrombin in tumor angiogenesis, implantation, and metastasis. Pathophysiol Haemost Thromb. 2003;33(suppl 1):54–5. doi: 10.1159/000073294. [DOI] [PubMed] [Google Scholar]
- 14.Ochmanski W. Influence of antiplatelet drugs (AD) on the efectiveness of combined therapy of small cell lung cancer. Part II. Influence of treatment on time of remission and patients survival. Przegl Lek. 2008;65:321–8. [Article in Polish] [PubMed] [Google Scholar]
- 15.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 16.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 17.Michelson AD, Frelinger AL, 3rd, Furman MI. Current options in platelet function testing. Am J Cardiol. 2006;98:4N–10N. doi: 10.1016/j.amjcard.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 19.Bidard FC, Hajage D, Bachelot T, et al. Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: a prospective observational study. Breast Cancer Res. 2012;14:R29. doi: 10.1186/bcr3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13:688–95. doi: 10.1016/S1470-2045(12)70209-7. [DOI] [PubMed] [Google Scholar]
- 21.Franken B, de Groot MR, Mastboom WJ, et al. Circulating tumor cells, disease recurrence and survival in newly diagnosed breast cancer. Breast Cancer Res. 2012;14:R133. doi: 10.1186/bcr3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakewell SJ, Nestor P, Prasad S, et al. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci U S A. 2003;100:14205–10. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engebraaten O, Trikha M, Juell S, et al. Inhibition of in vivo tumour growth by the blocking of host alpha(v)beta3 and alphaII(b)beta3 integrins. Anticancer Res. 2009;29:131–7. [PubMed] [Google Scholar]
- 24.Uluckan O, Eagleton MC, Floyd DH, et al. APT102, a novel adpase, cooperates with aspirin to disrupt bone metastasis in mice. J Cell Biochem. 2008;104:1311–23. doi: 10.1002/jcb.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosetti C, Gallus S, La Vecchia C. Aspirin and cancer risk: a summary review to 2007. Recent Results Cancer Res. 2009;181:231–51. doi: 10.1007/978-3-540-69297-3_22. [DOI] [PubMed] [Google Scholar]
- 26.Gann PH, Manson JE, Glynn RJ, et al. Low-dose aspirin and incidence of colo-rectal tumors in a randomized trial. J Natl Cancer Inst. 1993;85:1220–4. doi: 10.1093/jnci/85.15.1220. [DOI] [PubMed] [Google Scholar]
- 27.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin inthe primaryprevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 28.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–13. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 29.Lipton A, Scialla S, Harvey H, et al. Adjuvant antiplatelet therapy with aspirin in colo-rectal cancer. J Med. 1982;13:419–29. [PubMed] [Google Scholar]
- 30.Nieminen U, Kauppila A, Gronroos M, et al. Placebo-controlled study on the efficacy of the pyrimido-pyrimidine derivative RA 233 in ovarian cancer. Gynecol Oncol. 1990;36:226–31. doi: 10.1016/0090-8258(90)90179-o. [DOI] [PubMed] [Google Scholar]