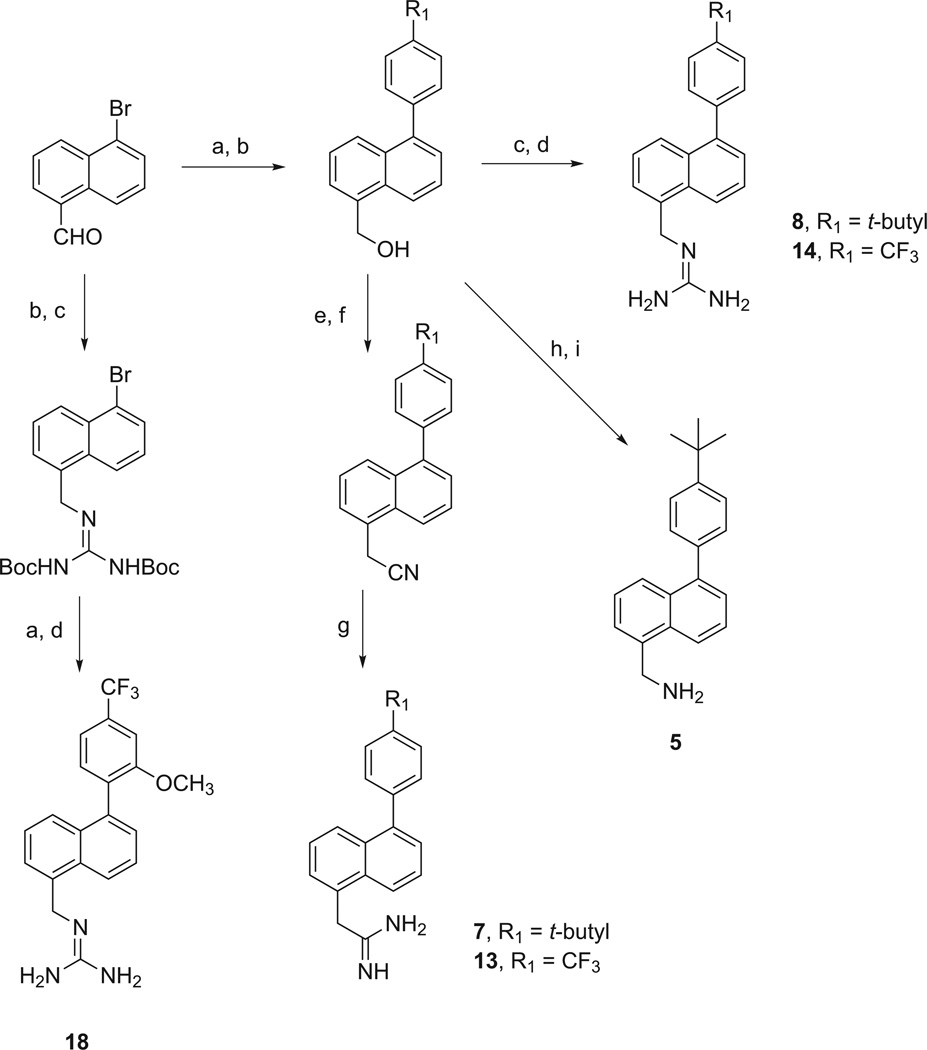
Scheme 2.
Methods used in the preparation of 5-substituted 1-(4-t-butylphenyl)naphthalenes, 5, 7, and 8, and 1-(4-trifluoromethylphenyl)naphthalene derivatives, 13, 14, and 18. Reagents and conditions: (a) R1R2-phenylboronic acid, Pd(OAc)2, XPhos, K2CO3, ACN/H2O, 90 °C; (b) NaBH4, EtOH, rt; (c) 1,3-bis(t-butoxycarbonyl)guanidine, PPh3, DIAD, toluene, 0 °C to rt; (d) TFA/CH2Cl2, 0 °C to rt; (e) PBr3, CH2Cl2, 0 °C to rt; (f) KCN, DMF, rt; (g) (i) 4 N HCl/dioxane, Et2O, MeOH, 0 °C; (ii) NH4Cl, EtOH, 85 °C [26]; (h) NaN3, PPh3, DMF/CCl4, 90 °C; (i) polymer-bound PPh3, THF/H2O, rt.