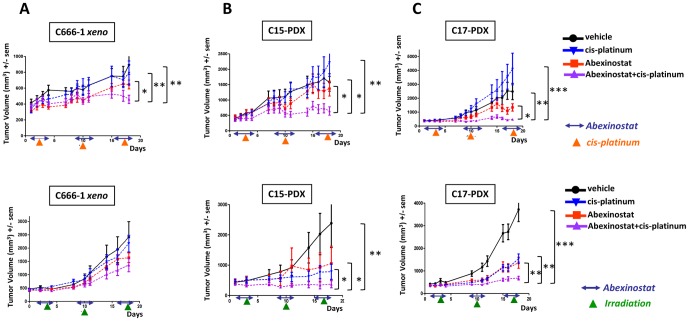
Figure 5. Cooperative cytotoxic effects of Abexinostat combined with CDDP or external irradiation on xenografted NPC tumors.
Treatment was started 12 days following subcutaneous graft of tumor fragments (350 mg per mouse). Groups of 10 mice were used for each therapeutic condition (vehicle−Abexinostat−vehicle + CDDP or irradiation−Abexinostat + CDDP or irradiation). Abexinostat was given from D1 to D4, D8 to D11 and D15 to D17 (two intra-peritoneal (IP) injections of 12.5 mg/kg per day with an interval of 6 to 8 hours). CDDP was given IP at a dose of 2 mg/kg at D3, D10 and D17 (upper panel). Selective irradiation of the tumor volume was done at D3, D10 and D17 (lower panel). Caliper tumor measurements and weighing of the animals were done at least 3 days a week. Mice were sacrificed at day 18 following the onset of the treatment. Error bars are based on SEM. A - Cooperative tumor growth reduction of C666-1 NPC xenografts when treated with Abexinostat combined with CDDP but not with irradiation. Tumor growth reduction is statistically significant at day 18 for the combination of Abexinostat with CDDP by comparison with tumors treated with the vehicle, Abexinostat alone or CDDP alone (upper graph). The combination of Abexinostat with irradiation has no statistically significant effects using this NPC model (lower graph). B - Cooperative tumor growth reduction of C15 NPC xenografts treated with Abexinostat combined with CDDP or irradiation. Tumor growth reductions are statistically significant for the combinations of Abexinostat with CDDP (upper graph) or with irradiation (lower graph) by comparison with tumors treated with the vehicle, Abexinostat, CDDP or irradiation used as single agents. C - Cooperative tumor growth reduction of C17 NPC xenografts treated with Abexinostat combined with CDDP or irradiation. Tumor growth reductions are statistically significant for the combinations of Abexinostat with CDDP (upper graph) or with irradiation (lower graph) by comparison with tumors treated with the vehicle, Abexinostat, CDDP or irradiation used as a single agent.