Abstract
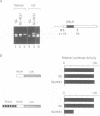
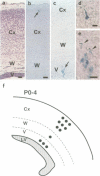
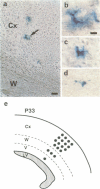
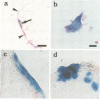
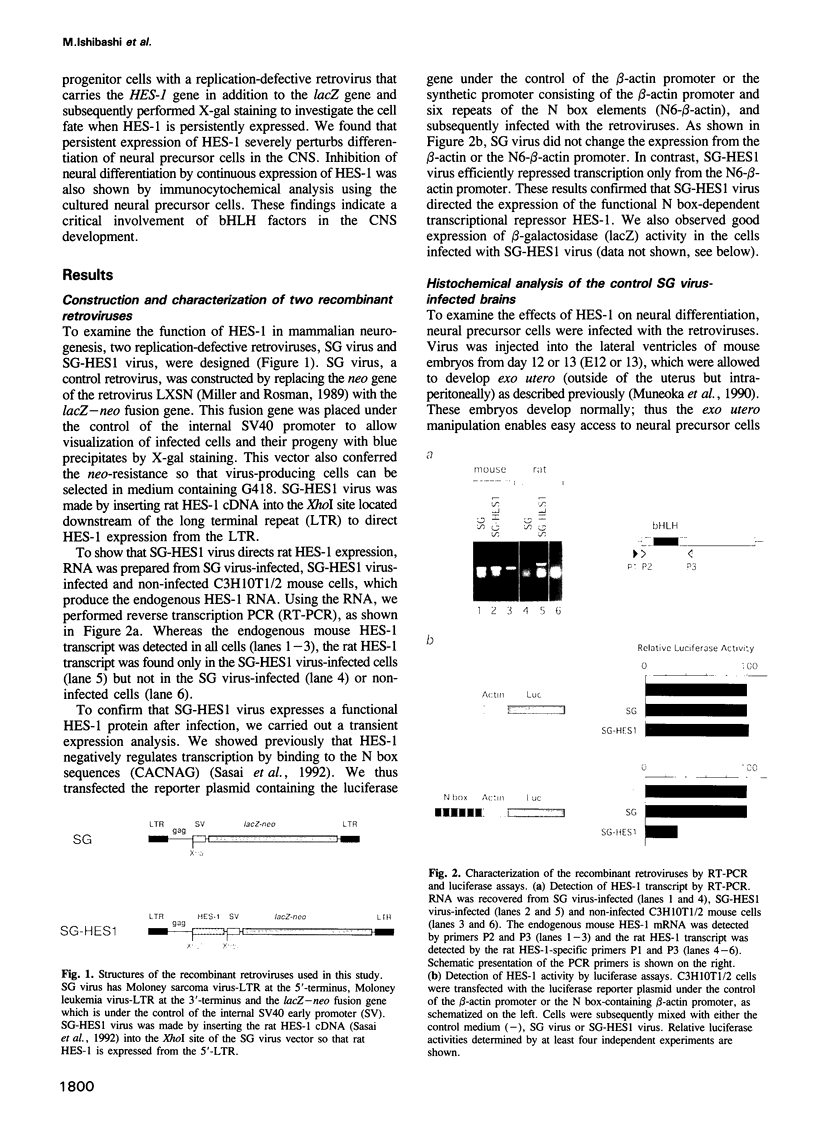
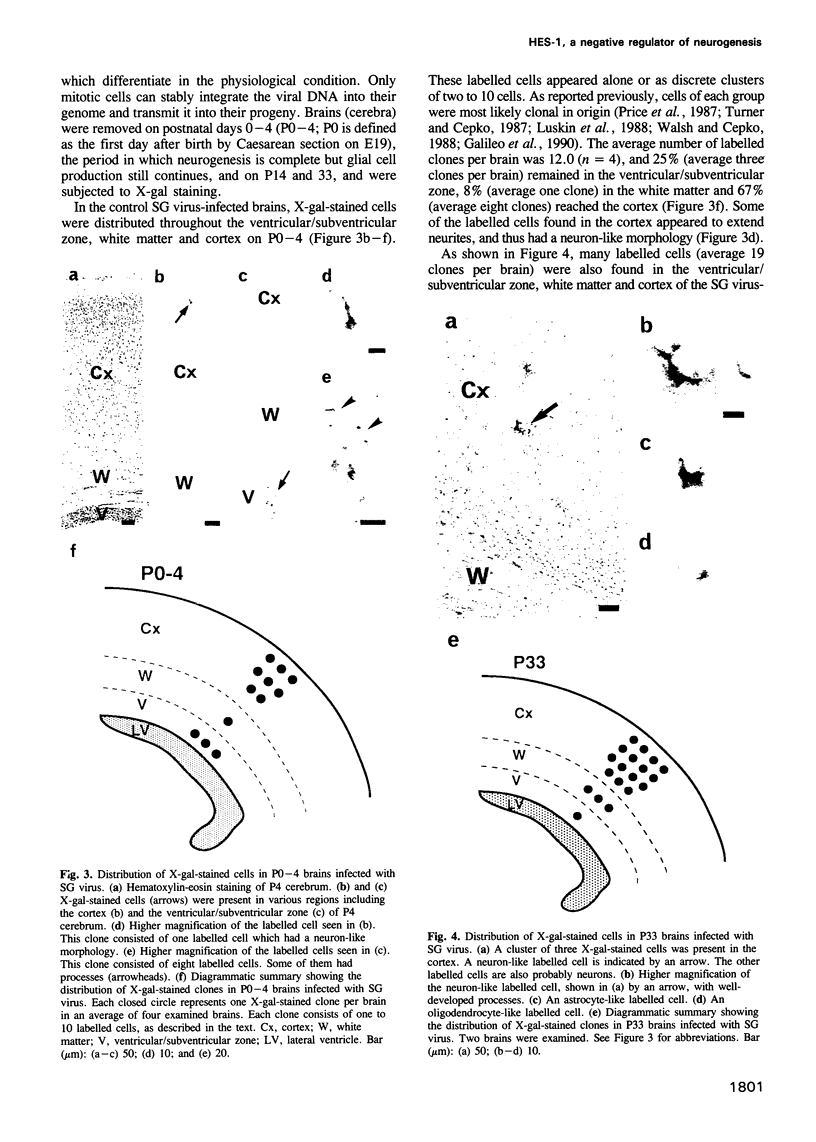
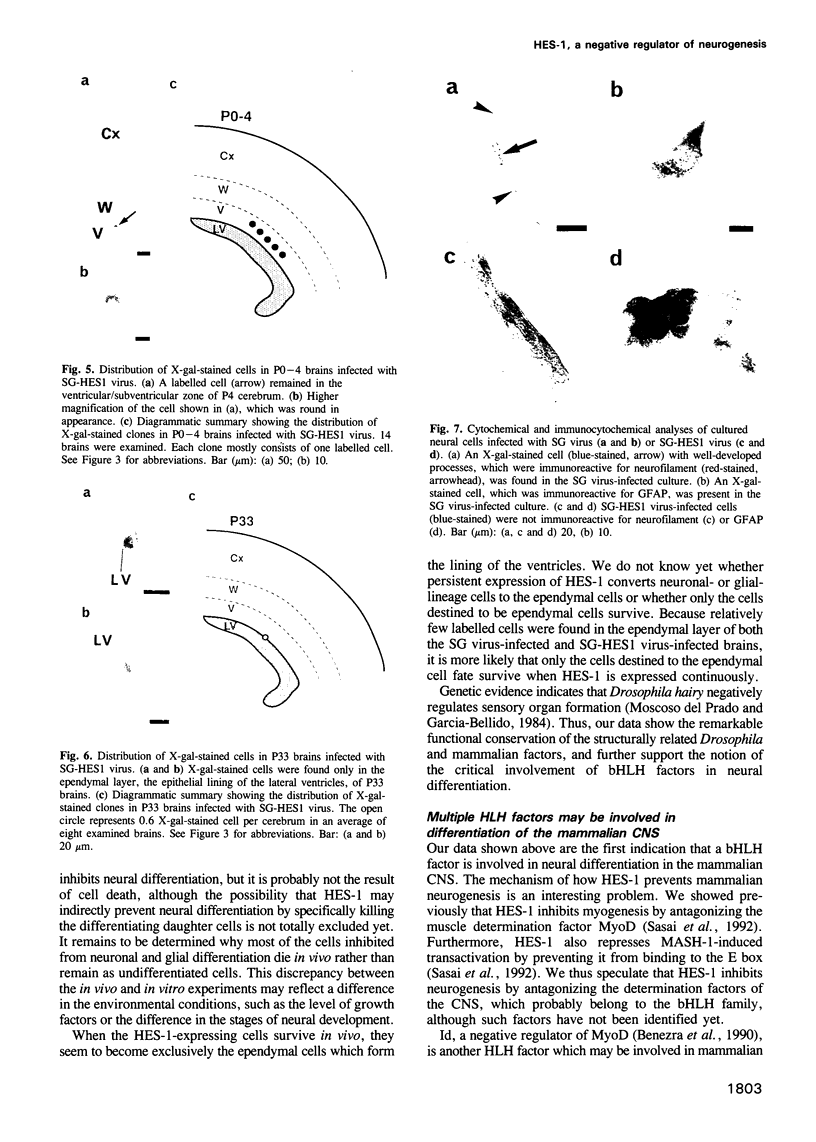
In the developing mammalian central nervous system, neural precursor cells present in the ventricular zone determine their fate to become neurons or glial cells, migrate towards the outer layers and undergo terminal differentiation. The transcriptional repressor HES-1, a basic helix-loop-helix (bHLH) factor structurally related to the Drosophila hairy gene, is expressed at high levels throughout the ventricular zone, but the level decreases as neural differentiation proceeds. Because of this negative correlation, we tested whether continuous expression of HES-1 inhibits neural differentiation. A HES-1 and lacZ-transducing retrovirus (SG-HES1) and a control lacZ-transducing retrovirus (SG) were injected into the lateral ventricles of mouse embryos, and the fate of the infected neural precursor cells was examined by X-gal staining. The SG virus-infected cells migrated and differentiated into neurons and glial cells. In contrast, the cells infected with SG-HES1 virus remained in the ventricular/subventricular zone, decreased to approximately 10% in number as compared with that of the newborn during the postnatal 4-5 weeks and, when they survived, were present exclusively in the ependymal layer. Furthermore, whereas cultured neural precursor cells infected with SG virus became immunoreactive for neuronal and glial markers, the cells infected with SG-HES1 virus did not. These results show that persistent expression of HES-1 severely perturbs neuronal and glial differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akazawa C., Sasai Y., Nakanishi S., Kageyama R. Molecular characterization of a rat negative regulator with a basic helix-loop-helix structure predominantly expressed in the developing nervous system. J Biol Chem. 1992 Oct 25;267(30):21879–21885. [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega J. A., Jan Y. N. Genetic and molecular bases of neurogenesis in Drosophila melanogaster. Annu Rev Neurosci. 1991;14:399–420. doi: 10.1146/annurev.ne.14.030191.002151. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Modolell J. Patterning of the Drosophila nervous system: the achaete-scute gene complex. Trends Genet. 1992 Jun;8(6):202–208. doi: 10.1016/0168-9525(92)90234-u. [DOI] [PubMed] [Google Scholar]
- Duncan M., DiCicco-Bloom E. M., Xiang X., Benezra R., Chada K. The gene for the helix-loop-helix protein, Id, is specifically expressed in neural precursors. Dev Biol. 1992 Nov;154(1):1–10. doi: 10.1016/0012-1606(92)90042-f. [DOI] [PubMed] [Google Scholar]
- Ellis H. M., Spann D. R., Posakony J. W. extramacrochaetae, a negative regulator of sensory organ development in Drosophila, defines a new class of helix-loop-helix proteins. Cell. 1990 Apr 6;61(1):27–38. doi: 10.1016/0092-8674(90)90212-w. [DOI] [PubMed] [Google Scholar]
- Feder J. N., Jan L. Y., Jan Y. N. A rat gene with sequence homology to the Drosophila gene hairy is rapidly induced by growth factors known to influence neuronal differentiation. Mol Cell Biol. 1993 Jan;13(1):105–113. doi: 10.1128/mcb.13.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich G., Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991 Sep;5(9):1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Galileo D. S., Gray G. E., Owens G. C., Majors J., Sanes J. R. Neurons and glia arise from a common progenitor in chicken optic tectum: demonstration with two retroviruses and cell type-specific antibodies. Proc Natl Acad Sci U S A. 1990 Jan;87(1):458–462. doi: 10.1073/pnas.87.1.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrell J., Modolell J. The Drosophila extramacrochaetae locus, an antagonist of proneural genes that, like these genes, encodes a helix-loop-helix protein. Cell. 1990 Apr 6;61(1):39–48. doi: 10.1016/0092-8674(90)90213-x. [DOI] [PubMed] [Google Scholar]
- Guillemot F., Lo L. C., Johnson J. E., Auerbach A., Anderson D. J., Joyner A. L. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993 Nov 5;75(3):463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Halliday A. L., Cepko C. L. Generation and migration of cells in the developing striatum. Neuron. 1992 Jul;9(1):15–26. doi: 10.1016/0896-6273(92)90216-z. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Sasai Y., Nakanishi S., Kageyama R. Molecular characterization of HES-2, a mammalian helix-loop-helix factor structurally related to Drosophila hairy and Enhancer of split. Eur J Biochem. 1993 Aug 1;215(3):645–652. doi: 10.1111/j.1432-1033.1993.tb18075.x. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell. 1993 Dec 3;75(5):827–830. doi: 10.1016/0092-8674(93)90525-u. [DOI] [PubMed] [Google Scholar]
- Jen Y., Weintraub H., Benezra R. Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev. 1992 Aug;6(8):1466–1479. doi: 10.1101/gad.6.8.1466. [DOI] [PubMed] [Google Scholar]
- Johnson J. E., Birren S. J., Anderson D. J. Two rat homologues of Drosophila achaete-scute specifically expressed in neuronal precursors. Nature. 1990 Aug 30;346(6287):858–861. doi: 10.1038/346858a0. [DOI] [PubMed] [Google Scholar]
- Kitani H., Shiurba R., Sakakura T., Tomooka Y. Isolation and characterization of mouse neural precursor cells in primary culture. In Vitro Cell Dev Biol. 1991 Aug;27A(8):615–624. doi: 10.1007/BF02631104. [DOI] [PubMed] [Google Scholar]
- Klämbt C., Knust E., Tietze K., Campos-Ortega J. A. Closely related transcripts encoded by the neurogenic gene complex enhancer of split of Drosophila melanogaster. EMBO J. 1989 Jan;8(1):203–210. doi: 10.1002/j.1460-2075.1989.tb03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo L. C., Johnson J. E., Wuenschell C. W., Saito T., Anderson D. J. Mammalian achaete-scute homolog 1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev. 1991 Sep;5(9):1524–1537. doi: 10.1101/gad.5.9.1524. [DOI] [PubMed] [Google Scholar]
- Luskin M. B., Pearlman A. L., Sanes J. R. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988 Oct;1(8):635–647. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Ow D. W., DE Wet J. R., Helinski D. R., Howell S. H., Wood K. V., Deluca M. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science. 1986 Nov 14;234(4778):856–859. doi: 10.1126/science.234.4778.856. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow C. A., Hogan A., Pinchin S. M., Howe K. M., Lardelli M., Ish-Horowicz D. The Drosophila hairy protein acts in both segmentation and bristle patterning and shows homology to N-myc. EMBO J. 1989 Oct;8(10):3095–3103. doi: 10.1002/j.1460-2075.1989.tb08461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y., Kageyama R., Tagawa Y., Shigemoto R., Nakanishi S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev. 1992 Dec;6(12B):2620–2634. doi: 10.1101/gad.6.12b.2620. [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Carroll S. B. Regulation of achaete-scute gene expression and sensory organ pattern formation in the Drosophila wing. Genes Dev. 1991 Jun;5(6):984–995. doi: 10.1101/gad.5.6.984. [DOI] [PubMed] [Google Scholar]
- Takebayashi K., Sasai Y., Sakai Y., Watanabe T., Nakanishi S., Kageyama R. Structure, chromosomal locus, and promoter analysis of the gene encoding the mouse helix-loop-helix factor HES-1. Negative autoregulation through the multiple N box elements. J Biol Chem. 1994 Feb 18;269(7):5150–5156. [PubMed] [Google Scholar]
- Tietze K., Oellers N., Knust E. Enhancer of splitD, a dominant mutation of Drosophila, and its use in the study of functional domains of a helix-loop-helix protein. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6152–6156. doi: 10.1073/pnas.89.13.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. L., Cepko C. L. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987 Jul 9;328(6126):131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Walsh C., Cepko C. L. Clonally related cortical cells show several migration patterns. Science. 1988 Sep 9;241(4871):1342–1345. doi: 10.1126/science.3137660. [DOI] [PubMed] [Google Scholar]