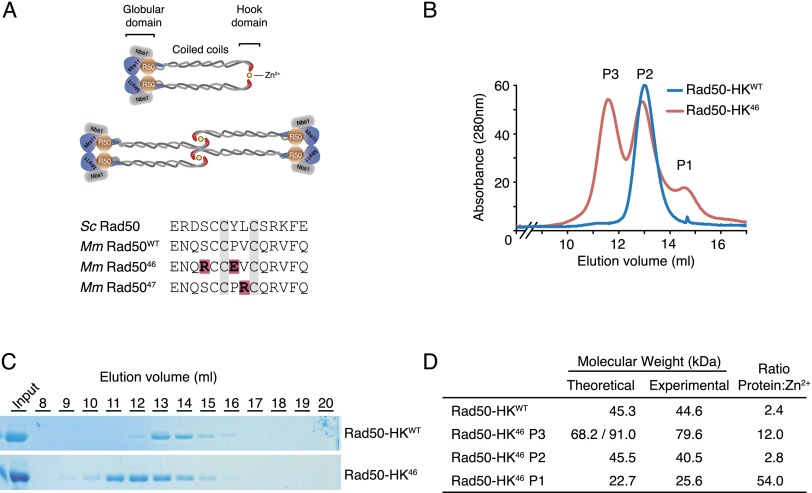
Figure 1.
Analysis of Rad50 hook dimerization. (A, top panel) Schematic representation of the Mre11 complex in two possible configurations. Rad50 globular domain shown in brown, Mre11 is in blue, and Nbs1 is in gray. (Bottom panel) Sequence of the hook domain from S. cerevisiae, Mus musculus, and the Rad5046 and Rad5047 mutants. (B) Gel filtration profile of Rad50-HK proteins. (C) Fractions from B were resolved on an acrylamide gel and stained with Coomassie. (D) Molecular weight and Zn2+ content of the indicated Rad50-HK proteins determined by SEC-MALS and ICP-MS, respectively. Theoretical molecular weight for a monomeric (P1), dimeric (P2 and Rad50-HKWT), and trimeric/tretrameric (P3) species.