Abstract
Recent evidence suggests that human papillomavirus (HPV)-positive head and neck squamous cell carcinoma (HNSCC) patients have better survival than HPV-negative patients. However, it is unclear if similar patterns for survival exist across different tumor sites, and whether HPV-associated prognosis is modified by type of treatment. We prospectively tested 222 histologically confirmed HNSCC primary tumors for HPV DNA by PCR and HPV E6/E7 RNA by RT-PCR prior to treatment at a large urban health center. Cox proportional hazard ratio models were constructed to assess HPV-associated differences in overall and disease-specific survival adjusting for clinical and demographic covariates. HPV detection varied significantly by primary HNSCC tumor site, from 35 % for oropharynx, to 25 % for hypopharynx, 5 % for larynx, and 3 % for oral cavity (p < 0.0001), with HPV16 accounting for the majority (95 %) of HPV-positive tumors. The hazard-risk of overall and disease-specific death comparing HPV16-positive versus negative oropharyngeal HNSCC was reduced by 74 and 89 %, respectively (p values < 0.05), and was independent of other prognostic indicators; no statistically significant changes in outcomes were observed for non-oropharyngeal HNSCC sites. Prediction of overall survival was better with combined DNA and RNA HPV16 positive PCR detection. There was no difference in HPV16-associated survival whether patients received either surgery or (chemo)radiotherapy as their initial treatment modality. Improved HPV-associated HNSCC survival is limited to patients with oropharyngeal primaries. No selective treatment advantage is observed for HPV-positive tumors, although clinical trials are needed to evaluate which treatment modalities provide the most benefit for HPV-positive HNSCC.
Electronic supplementary material
The online version of this article (doi:10.1007/s12105-013-0486-4) contains supplementary material, which is available to authorized users.
Keywords: Human papillomavirus, Head and neck neoplasms, Radiotherapy, Chemotherapy, Surgery
Introduction
Cancers of the head and neck constitute an anatomically heterogeneous group arising most often from the oral cavity, oropharynx, hypopharynx, and larynx. Human papillomavirus (HPV)-positive head and neck squamous cell carcinoma (HNSCC) is increasingly recognized as a distinct disease associated with improved survival and response to therapy [1–4]. HPV DNA detected in these tumors (predominantly HPV type 16, the most common high-risk type also found in cervical cancer) is frequently integrated and transcriptionally active, with the majority expressing viral oncoproteins E6 and E7 [5, 6]. In comparison to HPV16-negative tumors, HPV16-positive HNSCCs are more likely to be located within the oropharynx, exhibit non-keratinizing morphology and are diagnosed at advanced stage.
Although significant reductions in the risk of overall death and disease progression have been observed for HPV16-positive tumors [7] much of the evidence supporting a prognostic role of HPV to date has focused on oropharyngeal primaries, where HPV DNA is most commonly found [4]. Few studies have reported an association between HPV and HNSCC of the larynx [8, 9] and other subsites [7]. Methods for testing tumor tissue for HPV are not standardized [10] and, moreover, there is little consensus on optimal treatment for HPV-associated HNSCC. Recent studies have suggested favorable outcomes for HPV-positive HNSCC after surgery [11], radiation therapy [12], and chemoradiation regimens [13, 14].
In the present study, we sought to: (1) examine whether HPV detection in general and HPV16 specifically is associated with improved survival outcomes for patients with oropharyngeal and non-oropharyngeal HNSCC, (2) examine whether the association with survival is similar for HPV DNA and RNA detection, and (3) explore whether HPV-associated survival differs by type of initial therapy, where choice of therapy was based on tumor stage and other clinical characteristics, and not HPV status.
Materials and Methods
Study Population
The study cohort consisted of 225 prospectively enrolled patients with 230 total primary HNSCC (5 patients with multiple primaries). Of the 230 primary tumors, 222 were treated at Montefiore Medical Center (MMC) since 2002 (217 patients). Following histologic confirmation, stage was determined based on the American Joint Committee on Cancer classification (6th Edition), and detailed clinical and pathologic data, including information on smoking history and alcohol consumption, were collected by medical interview. Institutional Review Boards at MMC and the Albert Einstein College of Medicine approved the study protocol and all patients provided written informed consent.
Tumor samples and tissues were collected by surgical resection or biopsy prior to therapy, and snap frozen in liquid nitrogen immediately. Frozen tissue was screened by the attending pathologist for the presence of tumor cells and the remaining tumor tissue homogenized prior to DNA and RNA extraction.
HPV DNA Detection and Genotyping by PCR
Genomic DNA was isolated using the DNEasyTissue Kit (Qiagen; Valencia, CA). Presence of HPV-DNA was assessed using MY09/MY11/HMB01-PCR system with Gold AmpliTaq that amplifies a conserved 450 base-pair segment in the L1-sequence of HPV [15], plus nested-PCR using primers specific for the HPV16-E6 gene and the upstream regulatory region [16]. Samples testing positive by consensus PCR were further genotyped using biotinylated oligonucleotide probes for 55 HPV types that include HPV16 and 12 established high-risk (oncogenic) types [17] and other low-risk types [15]. A complete set of controls was included to verify the specificity of the hybridizations. The integrity of specimen DNA was verified by amplification of a fragment of the ß-globin gene. In addition, HPV-positive and negative control samples were included in each PCR using DNA from a cervical carcinoma cell-line (SiHa) with two copies of HPV16 per cell [18] and a HPV-negative cell line HepG2.
HPV16 E6 and E7 mRNA Detection
We also tested for HPV16 RNA expression by reverse transcription PCR (RT-PCR). Total RNA was extracted from the same or matched frozen tumors samples in TRIzol by standard protocol (Invitrogen; Carlsbad, CA). RNA extractions were tested for HPV16 transcripts using oligonucleotide primers that span the 204 to 525 base-pair regions of the E6 and E7 oncogenes [19, 20]. The primers were designed to amplify full-length HPV16-E6 mRNA and its splice variant. HPV16-E7 primers were designed based on sequence NC_001526 (complete genome) using the web-based Primer3 design program (available at http://frodo.wi.mit.edu/primer3). GAPDH primers were included in each assay as an RNA control; designed based on sequence AY340484 (Homo sapiens glyceraldehyde-3-phosphate dehydrogenase gene, complete cds). HPV16 RNA-positive tumors were defined as those that expressed either E6 or E7 transcripts, and RNA-negative if they only expressed GAPDH. Preliminary review of 15 discordant samples by HPV16 DNA and RNA detection suggested potential contamination during homogenization of tissue. To address this, RNA and DNA were co-extracted from 11 to 15 formalin-fixed paraffin-embedded tissue blocks of potentially contaminated samples and retested using real-time PCR and coded based on the retest results. Further sensitivity analyses excluding these samples or assigning them HPV16 negative did not appreciably change the main effect estimates in the current study.
Follow-up and Ascertainment of Clinical Outcome
Participating patients were followed prospectively for up to 113 months (median followup = 60 months) from time of diagnosis. Detailed treatment information (on primary surgery, radiation and chemotherapy), and evidence of disease following treatment, ascertained by clinical exam, PET/CT imaging and pathological evaluation, was collected for all patients. Overall survival was defined as time from diagnosis (in months) to death from all causes. Disease-specific survival was defined as the time to death from disease, and locoregional recurrence as the time to either local or regional recurrence of disease. These endpoints were selected as having widespread acceptance and relevance for HNSCC [21].
Statistical Analyses
We restricted the analysis to primary tumors that included invasive HNSCC of the oropharynx (base of tongue, tonsil, soft palate, oropharyngeal wall, uvula and oropharynx-not otherwise specified [NOS]), hypopharynx (posterior pharyngeal wall, pyriform sinus and hypopharynx-NOS), larynx (glottis, supraglottis-aryepiglottic fold, supraglottis-false vocal cord, supraglottis-arytenoid, supraglottis-epiglottis, supraglottis-NOS and larynx-NOS), and oral cavity (buccal, alveolar ridge, anterior tongue, floor of mouth, hard palate, inner lip mucosa and retromolar trigone). Tumors were also grouped into oropharyngeal and non-oropharyngeal cancers; subgroup analyses showed similar adjusted survival for non-oropharyngeal sites considered HPV16 positive, which provided a basis for categorization. HPV16 DNA and RNA positive tumors were also grouped together for purpose of analysis. Under this combined definition, tumors were classified as HPV16 negative if they were negative for either DNA or RNA.
Differences in HPV16 detection prevalence by clinical and demographic characteristics were examined by contingency tables and non-parametric tests. To evaluate associations between HPV16 detection with survival, we generated Kaplan–Meier plots and constructed Cox proportional hazards regression models adjusting for strong prognostic indicators and confounders. Confounding factors were identified using a change in point estimate criterion [22], and were subsequently controlled for in the multivariable regression models. The potential for confounding was examined for all socio-demographic and clinical indicators: age, gender, race, ethnicity, smoking history (including comparisons: never versus ever, former and by pack-years), alcohol consumption, tumor anatomic site, tumor stage, primary treatment modality, method of specimen procurement (biopsy vs. surgical or laser resection), eastern cooperative oncology group (ECOG) performance status [23], and tumor location (bilateral vs. unilateral). Additionally, we tested for multi-collinearity between model variables by examining inter-correlations between independent covariates using variance inflation factors and tolerance values [24]. Proportional hazards assumptions were confirmed by inspection of adjusted log [-log (survival)] curves and examination of time-dependent covariates [25]. Time-dependent covariates were stratified in multivariable models.
To evaluate differences in HPV16 survival between tumor sites, we tested for effect modification by fitting the multivariable models with the cross-product term of HPV16 and tumor site (oropharynx/non-oropharynx). Inference was based on the Wald χ2 test statistic for two-way interaction. To assess whether the association between HPV infection and survival differed by primary treatment modality, we constructed separate stratified models by initial treatment based upon intent to treat, comparing: (a) HPV16 by primary surgery with and without adjuvant therapy and (b) HPV16 by primary nonsurgical therapy, defined as either primary radiotherapy or chemoradiotherapy with or without salvage neck dissection. Each treatment-stratified model was further adjusted for the cross-product term of HPV16 and tumor site to test for effect modification by tumor site. HPV status was not taken into account for treatment decisions, and all patients were prospectively discussed at a multidisciplinary tumor conference. Statistical analyses were conducted with the SAS 9.2 statistical software package (Cary, NC), and all tests were two-sided.
Results
Two hundred seventeen (217) patients contributed 222 primary HNSCC tumor samples for analysis; of these, 176 samples had sufficient genetic material for HPV DNA detection and 203 for HPV16 RNA detection by PCR. Patients were largely White (62.2 %), non-Hispanic (68.9 %), male (72.9 %), and aged 62 ± 12 years (mean ± standard deviation). Fifty-one (27.9 %) tumors tested positive for any HPV DNA type. Of these, seven different HPV types were identified, three of which were high-risk types (HPV16: 83 %; HPV33: 2 %; HPV35: 2 %). HPV33 and 35 were found exclusively in the oropharynx (HPV33 in the base of tongue and HPV35 in the tonsil). Low-risk types detected included HPV−32/42, 53, 72, and other uncharacterized HPV types, i.e., PCR positive, type unknown, amounting to a combined prevalence of 11.5 %. HPV type co-infections were observed in one sample positive for both HPV16 and 72.
Given HPV16 was detected in the vast majority (96 %, N = 43) of high-risk HPV-positive cases by MY09/MY11/HMB01-PCR, we assessed clinical associations in relation to HPV16 DNA and RNA status. HPV16 E6/E7-RNA expression was detected in 31 % of tumors tested (N = 63/203). Overlap between HPV16 DNA and RNA detection, assessed in 162 tumors, was substantial (kappa statistic for agreement = 0.67, 95 % confidence interval [CI] 0.55–0.80). Using the combined definition for HPV16 status previously described, a total of 32 HPV16-positive and 176 HPV16-negative cases were identified, for a total of 208 HNSCC with HPV DNA and RNA results.
Overall, HPV16 prevalence using our combined definition varied significantly by anatomic site with a higher prevalence observed in the oropharynx (35 %) as compared to the hypopharynx (29 %), larynx (5 %) and oral cavity (3 %; p < 0.0001). Overall, a majority of patients were current or former smokers (85 %), with a mean of 52 pack-years of smoking (standard deviation = 37; range = 0.20–245). In the oropharynx, HPV16-positive patients were more likely to be never smokers or never drinkers, and present with a higher nodal stage than HPV16-negative cases (Table 1). Moreover, the average number of pack-years of smoking was significantly lower in HPV16-positive as compared to negative patients with oropharyngeal tumors (36 vs. 62; p < 0.01). In the non-oropharynx, HPV16 was more likely to be detected in the hypopharynx (p = 0.01) and HPV16-positive patients were largely never/former smokers.
Table 1.
Study population characteristics stratified by HPV16 statusa and anatomical site in the overall population
| Oropharynx (N = 65) | Non-oropharynx (N = 143) | |||||
|---|---|---|---|---|---|---|
| HPV16 Negative N = 42 | HPV16 Positive N = 23 | p valueb | HPV16 Negative N = 134 | HPV16 Positive N = 9 | p valueb | |
| Patient Demographics | ||||||
| Age at diagnosis in years | ||||||
| Mean (standard deviation) | 64 (10) | 59 (8) | 0.18 | 62 (13) | 68 (14) | 0.59 |
| Range | 42–84 | 40–76 | 22–89 | 51–91 | ||
| Sex, n (%) | ||||||
| Men | 34 (81 %) | 16 (70 %) | 0.36 | 92 (69 %) | 7 (78 %) | 0.72 |
| Women | 8 (19 %) | 7 (30 %) | 42 (31 %) | 2 (22 %) | ||
| Racec, n (%) | ||||||
| White/Asian | 23 (61 %) | 14 (64 %) | 1.00 | 87 (73 %) | 8 (89 %) | 0.44 |
| Black/African American | 15 (39 %) | 8 (36 %) | 33 (28 %) | 1 (11 %) | ||
| Ethnicityc, n (%) | ||||||
| Non-ispanic | 34 (83 %) | 15 (69 %) | 0.21 | 90 (70 %) | 7 (78 %) | 1.00 |
| Hispanic | 7 (17 %) | 7 (32 %) | 38 (30 %) | 2 (22 %) | ||
| Smoking statusd, n (%) | ||||||
| Current smoker | 20 (48 %) | 7 (30 %) | 0.14d | 53 (40 %) | 1 (11 %) | 0.04d |
| Former smoker | 17 (40 %) | 11 (48 %) | 62 (46 %) | 5 (56 %) | ||
| Never smoker | 5 (12 %) | 5 (22 %) | 19 (14 %) | 3 (33 %) | ||
| Drinking statusd, n (%) | ||||||
| Current drinker | 19 (45 %) | 7 (30 %) | 0.08d | 34 (25 %) | 1 (11 %) | 0.24d |
| Former Drinker | 7 (17 %) | 1 (4 %) | 22 (16 %) | 1 (11 %) | ||
| Never Drinker | 16 (38 %) | 15 (65 %) | 78 (58 %) | 7 (78 %) | ||
| Clinical characteristics | ||||||
| Anatomical sitee, n (%) | ||||||
| Oropharynx | 42 (100 %) | 23 (100 %) | – | – | ||
| Oral Cavity | – | – | 65 (49 %) | 2 (22 %) | 0.01 | |
| Larynx | – | – | 59 (44 %) | 3 (33 %) | ||
| Hypopharynx | – | – | 10 (7 %) | 4 (44 %) | ||
| Overall stage, n (%) | ||||||
| I–II | 13 (31 %) | 5 (22 %) | 0.57 | 32 (24 %) | 0 (0 %) | 0.21 |
| III–IV | 29 (69 %) | 18 (78 %) | 102 (76 %) | 9 (100 %) | ||
| Nodal stage n (%) | ||||||
| N0–N1 | 22 (52 %) | 7 (30 %) | 0.12 | 83 (62 %) | 6 (67 %) | 1.00 |
| N2–N3 | 20 (48 %) | 16 (70 %) | 51 (38 %) | 3 (33 %) | ||
| Tumor size n (%) | ||||||
| T1–T2 | 26 (62 %) | 17 (74 %) | 0.42 | 60 (45 %) | 2 (22 %) | 0.30 |
| T3–T4 | 16 (38 %) | 6 (26 %) | 74 (55 %) | 7 (78 %) | ||
| Primary tumor site no., n (%) | ||||||
| 1st | 35 (83 %) | 22 (96 %) | 0.24 | 117 (87 %) | 8 (89 %) | 1.00 |
| 2nd–4th | 7 (17 %) | 1 (4 %) | 17 (13 %) | 1 (11 %) | ||
| ECOG performance statusc, n (%) | ||||||
| 0–1 | 22 (71 %) | 19 (90 %) | 0.17 | 72 (66 %) | 6 (67 %) | 1.00 |
| ≥ 2 | 9 (29 %) | 2 (10 %) | 37 (34 %) | 3 (33 %) | ||
| Initial treatment, n (%) | ||||||
| Surgical | 16 (38 %) | 11 (48 %) | 0.60 | 88 (66 %) | 6 (67 %) | 1.00 |
| Non-surgicalf | 26 (62 %) | 12 (52 %) | 46 (34 %) | 3 (33 %) | ||
aHPV16 tumors were detected positive by both HPV DNA PCR and oncogenic expression of E6/E7 by RNA RTPCR (combined definition)
bP-value for 2-sided fisher exact test
cRow numbers may not sum to column totals due to missing data
dSmoking status was defined as never smoked, ex-smoker (at time of diagnosis), and current smoker. Drinking status was defined as never drank alcohol regularly for more than 1 year, former or current drinker (at time of diagnosis). p values for two-sided Cochrane-Armitage trend test
eOropharynx includes base of tongue, tonsil, soft palate, oropharyngeal wall, uvula, and oropharynx-NOS. Hypopharynx includes: posterior pharyngeal wall, pyriform sinus, and hypopharynx-NOS. Larynx includes: glottis, supraglottis-aryepiglottic fold, and epiglottis. Oral cavity includes: buccal, alveolar ridge, anterior tongue, floor of mouth, hard palate, inner lip mucosa, and retromolar trigone
fPrimary non-surgical therapy was defined as treatment with either primary radio-therapy with or without concomitant chemotherapy, including planned neck dissection and salvage surgery
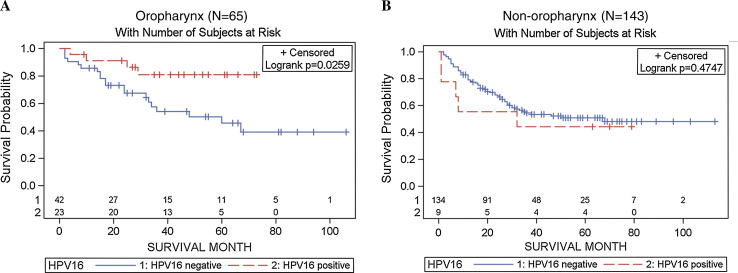
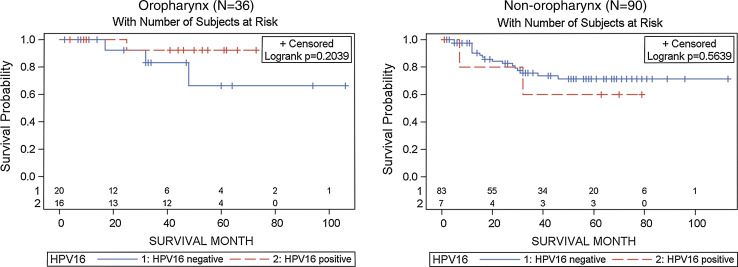
Kaplan–Meier plots showed markedly improved overall survival for HPV16-positive versus negative oropharyngeal cases (Log-rank p = 0.03; Fig. 1). In contrast, there were no differences in overall survival when non-oropharyngeal cases were considered. We observed similar differences with disease-specific survival, particularly among those who were either non-smokers or non-drinkers (Fig. 2). Further restriction of Kaplan–Meier plots to Hispanic patients demonstrated better HPV16-associated overall and disease-specific survival as well as time to locoregional recurrence, although not reaching statistical significance (data not shown).
Fig. 1.
Kaplan Meier plots for HPV16-associated overall survival stratified by tumor site Oropharynx (left panel), and non-oropharynx (right panel). HPV16 tumors were detected positive by both HPV DNA PCR and oncogenic expression of E6/E7 by RNA RT-PCR (combined definition)
Fig. 2.
Kaplan Meier (KM) plots for HPV-associated disease-specific survival among non-smokers or non-drinkers by tumor site Oropharynx (left panel), and non-oropharynx (right panel). HPV16 tumors were detected positive by both HPV DNA PCR and oncogenic expression of E6/E7 by RNA RT-PCR (combined definition)
Using our combined definition for HPV16 detection, there were significant differences in overall survival by tumor site (oropharynx vs. non-oropharynx; p for interaction = 0.04). After adjustment for strong prognostic indicators (age, tumor stage, nodal status and primary treatment), potential confounders (sex, tumor size, smoking and drinking), and an interaction term (HPV*tumor site), HPV16-positive oropharyngeal cases exhibited a significantly lower hazard-risk of overall death compared to HPV16-negative oropharyngeal cases, irrespective of method for HPV16 detection (Table 2). Hazard ratios were even stronger when disease-specific survival was considered. Additionally, we observed a lower, albeit non-significant, hazard-risk of locoregional recurrence (by 50 %) after 90 months of follow-up, comparing HPV16 positive with negative oropharyngeal tumors (p = 0.40; data not shown). These protective effects were attenuated for non-oropharyngeal cancers.
Table 2.
Overall and disease-specific survival by HPV16 DNA and RNA detection
| Overall N | Oropharynx | Non-oropharynx | |||||
|---|---|---|---|---|---|---|---|
| HPV16 (positive vs. negative) | n | Hazard ratiosa (95 % CI) | p value | n | Hazard ratiosa (95 % CI) | p value | |
| Overall survival | |||||||
| HPV16 DNAb | 176 | 57 | 0.37 (0.15–0.89) | 0.03 | 119 | 0.83 (0.32–2.13) | 0.70 |
| HPV16 RNAc | 203 | 67 | 0.35 (0.17–0.74) | <0.01 | 136 | 0.72 (0.36–1.44) | 0.35 |
| HPV16 combined definitiond | 208 | 65 | 0.26 (0.09–0.72) | 0.01 | 143 | 0.95 (0.37–2.43) | 0.91 |
| Disease-specific survival | |||||||
| HPV16 DNAb | 176 | 57 | 0.10 (0.01–0.84) | 0.03 | 119 | 0.51 (0.13–2.00) | 0.36 |
| HPV16 RNAc | 203 | 67 | 0.13 (0.03–0.61) | <0.01 | 136 | 0.59 (0.20–1.72) | 0.34 |
| HPV16 combined definitiond | 208 | 65 | 0.11 (0.01–0.86) | 0.03 | 143 | 0.65 (0.16–2.59) | 0.54 |
aHazard ratios adjusted for age, sex, nodal stage, tumor size, primary treatment, smoking, drinking, and interaction term (HPV16*tumor site). See Supplemental Table 1 for hazard ratios of all variables
bIncludes available data for HPV16 DNA detection only (79 % of total sample)
cIncludes available data for HPV16 RNA detection only (91 % of total sample)
dHPV16 is positive if DNA and RNA PCR detection are both positive. HPV16 is negative if either DNA or RNA PCR detection is negative. Missing data for RNA and DNA PCR detection is omitted
When we examined which subsites within and proximal to the oropharynx exhibited the strongest survival effects, patients with HPV16-positive tonsillar (n = 26) and base of tongue tumors (n = 23) had better overall survival than those with HPV16-negative tumors (log-rank p = 0.18 for Wald test; Supplemental Fig.1). After adjustment for nodal stage, primary treatment, and primary tumor number, the hazard-risk of dying was reduced by 48 % among those with HPV16-positive versus negative tumors (HR = 0.52, 95 % CI 0.15–1.77). No significant improvement in overall survival was observed for hypopharyngeal or supraglottic tumors; however, there were no deaths observed in patients with HPV16-positive supraglottic tumors over an 80-month follow-up period.
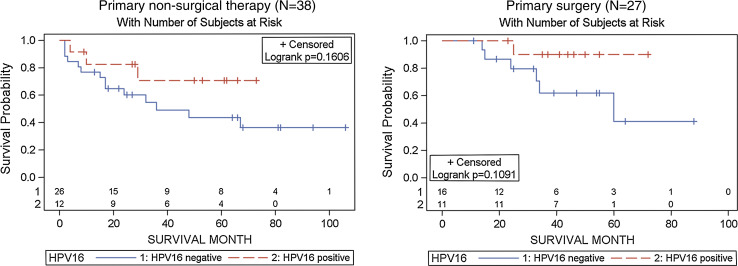
Lastly, to evaluate whether HPV16 modified the effect of treatment on overall survival, we first examined Kaplan–Meier plots stratified by primary treatment modality in the oropharynx: (a) surgery (with or without adjuvant radiotherapy or chemoradiotherapy), and (b) non-surgical therapy with radiation alone or combined with chemotherapy, based upon primary treatment. While not significant, HPV16 detection was associated with improved overall survival in patients receiving either surgical or non-surgical therapy as their primary treatment (Fig. 3). Similar non-significant associations were observed after adjustment for nodal stage, smoking and drinking (data not shown). No statistically significant differences were observed for HPV-associated survival by treatment modality in multivariable models (p for interaction = 0.81). While some patients required salvage surgery following therapy or received adjuvant therapy following surgery, a subset received concomitant therapy with surgery as part of a planned treatment regimen designated at diagnosis. Restricting observations to these 29 patients did not reveal substantively different HPV-associated survival estimates (data not shown).
Fig. 3.
Kaplan Meier plots for overall survival in oropharyngeal cancer patients by primary treatment Primary non-surgical therapy (left panel), and primary surgery (right panel). HPV16 tumors were detected positive by both HPV DNA PCR and oncogenic expression of E6/E7 by RNA RT-PCR (combined definition)
Discussion
Conventional treatment for HNSCC involves multidisciplinary interventions with initial treatment choice dependent upon disease location, extent of the primary tumor, and presence of metastatic disease, as well as patient comborbidities, expected functional outcomes of therapy and patient preferences. Advanced stage disease will generally be treated with multi-modality therapy, often employing chemotherapy and radiation, with or without surgery. However, any of these therapies can, and do, produce morbidities affecting speech, swallowing and overall quality of life. Demonstrating treatment-specific outcomes for HPV-positive HNSCC therefore has therapeutic implications.
Our results demonstrate an improvement in overall and disease-specific survival of HPV16 positive HNSCC which supports a growing consensus that HPV status is an independent predictor of survival. Overall, our estimates showed a 56 % (p = 0.03) reduction in risk of death overall for HPV16-positive compared to HPV16-negative HNSCC patients, after adjustment for tumor site and other prognostic factors and potential confounders. This is consistent with a recent meta-analysis of pooled data from 42 studies, which reported a 54 % better overall survival in HNSCC-positive versus negative patients [26]. In contrast, a meta-analysis of 23 case-series studies showed a 15 % reduction in risk of death in HPV-positive HNSCC patients compared to their HPV-negative counterparts [3]. Although that finding was unadjusted and likely underestimated, another study of 253 patients with newly diagnosed HNSCC showed a 40 % (p = 0.07) reduction in risk of death after adjustment for nodal status, age, and alcohol consumption [4].
Improvement in overall and disease-specific survival in our population was most consistently observed for oropharyngeal cancers, where HPV16 prevalence was highest. When we stratified for tumor site, there was a 74 % reduction in risk of overall death (p = 0.01), which is consistent with recent reports on oropharyngeal cancer [3, 14]. Moreover, we found even stronger protective associations for disease-specific events including cancer death for HPV16-positive oropharyngeal cancers compared to their HPV16-negative counterparts. A lower risk of secondary primary cancers has also been attributed to HPV-associated HNSCC [27]. Although a subset of cases included in this study presented with second or higher primary HNSCC, excluding or adjusting for subsequent primaries did not change the observed survival associations with HPV. Interestingly, we found that Hispanic patients presented with better HPV-associated overall and disease-specific survival than Non-Hispanic patients among those with oropharyngeal cancers. However, adjustment for strong prognostic factors and behavioral risk factors (smoking and drinking) removed the effect of ethnicity on survival.
The higher HPV prevalence observed in the oropharynx and proximal subsites relative to other HNSCC sites further adds to the body of evidence that supports a changing epidemiological profile of oropharyngeal malignancies. Recent surveys from the US and Western Europe indicate an increase in the incidence of oropharyngeal cancers, particularly those of the tonsil and base of tongue [28–30] accumulating evidence supports the role of HPV infections in driving these upward trends [31–35]. As reported by others [1] HPV-positive HNSCC tends to be of oropharyngeal origin, diagnosed at a later stage, and found in younger individuals.
Comparing HPV16 DNA and RNA detection by PCR, we observe a substantial concordance in DNA prevalence and E6 and E7 expression. However, the presence of E6/E7 RNA in HPV16 DNA negative tumors might suggest the existence of integrated virus with loss or disruption of the L1 open reading frame [36]. Following integration, the intact portion of the HPV16 genome can produce multiple E6 and E7 oncogene transcripts [37]. Comparisons between HPV16 DNA +/RNA- versus RNA +/DNA- tumors by PCR in our study revealed that the improvement in survival was slightly stronger for tumors positive for HPV16-E6/E7 RNA (data not shown) suggesting that detection of these oncogenes expressed in the tumor may be important in identifying HPV-associated HNSCC that exhibit better prognosis, although the numbers were too small to reach statistical significance. Tumors positive for both HPV16 DNA and RNA were also more likely to originate in the tonsil and base of tongue of the oropharynx supporting the site-specific nature of this disease. Similar findings have recently been reported by Shi et al. [38]. using RT-PCR and in situ hybridization assays to detect HPV RNA and DNA, respectively, in oropharyngeal HNSCC.
There remains much debate around the underlying mechanism that drives a better response to therapy for HPV-positive HNSCC. Some have attributed the improvement to an enhanced sensitivity to chemotherapy and radiation of HPV-positive tumors due to expression of significant amounts of functional p53 [39], whereas others suggest that administration of radiation may enhance immune response to viral antigens [40]. In the current study, HPV-associated survival did not differ significantly by treatment modality in the oropharynx. An earlier study reported higher rates of response to therapy for HPV-positive oropharyngeal tumors or those expressing p16 protein relative to their negative counterparts after treatment with either chemo-radiotherapy or induction chemotherapy [41]. The up-regulation of p16 has been suggested as a marker of active HPV infection in HNSCC [42] whereas inactivation of p53 and p16 by mutation or methylation may be a more common event in smoking associated cancers [43]. In another study of 57 oropharyngeal cancers, improved prognosis was observed among p16 positive versus negative cases initially treated with either radiotherapy or chemoradiotherapy (log-rank test p = 0.10) or by surgery with or without adjuvant radiotherapy (p = 0.07) [44]. A recent study similarly found improved overall survival in HPV-positive as compared to HPV-negative oropharyngeal cancers treated with radiotherapy, but found no improvement among those receiving no radiotherapy [45]. While estimates did not reach statistical significance, likely due to small numbers, our results showed an improvement in overall survival comparing HPV16 positive with HPV16 negative oropharyngeal cancers, irrespective of initial therapy. In our population, patients who received transoral robotic surgery (TORS; n = 11) were more likely to have lower nodal stage and tumor size. In sensitivity analyses, hazard-risks of overall death comparing HPV16-positive to HPV16-negative oropharyngeal tumors were unchanged when we excluded 8 patients who received TORS and adjusted for nodal stage and tumor size.
This is one of the first studies to compare HPV-associated survival by primary treatment modality in HNSCC. While previous studies have tested for HPV DNA or RNA alone, or used proxy measures such as p16 immunohistochemistry, we used PCR methodology to examine HPV prevalence and expression; incorporating a combined DNA and RNA definition, we were able to define transcriptionally active tumors rather than tumor HPV status alone [46]. However, we recognize that the design has some limitations. Because this is a non-random sample, we tested for potential selection bias due to treatment allocation by calculating and adjusting for a treatment propensity score using a variety of statistical approaches [47]. This score can be defined as the probability of a patient with specific diagnostic indicators receiving a given initial treatment [48, 49]. However, estimates did not differ significantly from propensity unadjusted estimates (data not shown) and we therefore only report the latter. Lastly, we had small numbers of HPV16 DNA and RNA positive cases in the non-oropharynx and were likely not sufficiently powered to detect a survival advantage for these patients. It is possible that transcriptionally-active HPV-positive HNSCC of the oral cavity, larynx and hypopharynx, while rare, may exhibit better survival than their HPV-negative counterparts, as illustrated by the hazard ratios for disease-specific survival on Table 2. Larger studies are necessary to confirm our findings.
In summary, we found that HPV in HNSCC tumors has important clinical implications in treatment management. In particular, we show a significantly better survival for patients with HPV-positive oropharyngeal cancers, irrespective of primary treatment. These findings provide impetus for clinical trials assessing specific therapies currently in progress. The use of transoral robotic surgery in the primary surgical management of HPV-positive HNSCC also remains to be clearly defined. Moreover, the recent development of radio-immunotherapies for virally associated cancers targeting HPV16-E6 oncoproteins [50] provide further encouragement for intervention and control of a substantial fraction of HNSCC.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank the participants of this study; Margaret Brandwein-Gensler for her help with pathological staging and processing of tissue specimens; Catherine Sarta for her time and effort spent enrolling participants and with data entry; Gregory Rosenblatt for his assistance with data management; and Leslie Adrien for her help preparing and handling the biospecimens for molecular analysis. This work is supported in part by the National Cancer Institute [grant number CA115243]; National Institute of Dental and Craniofacial Research [T32 training grant DE007255]; the Einstein Cancer Research Center (P30 grant CA013330); and the Departments of Otorhinolaryngology-Head and Neck Surgery and Pathology at Albert Einstein College of Medicine and Montefiore Medical Center.
References
- 1.Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 2.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 3.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 4.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 5.Lace MJ, Anson JR, Klussmann JP, Wang DH, Smith EM, Haugen TH, et al. Human papillomavirus type 16 (HPV-16) genomes integrated in head and neck cancers and in HPV-16-immortalized human keratinocyte clones express chimeric virus-cell mRNAs similar to those found in cervical cancers. J Virol. 2011;85(4):1645–1654. doi: 10.1128/JVI.02093-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafkamp HC, Speel EJ, Haesevoets A, Bot FJ, Dinjens WN, Ramaekers FC, et al. A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5–8. Int J Cancer. 2003;107(3):394–400. doi: 10.1002/ijc.11389. [DOI] [PubMed] [Google Scholar]
- 7.Syrjanen S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005;32(Suppl 1):S59–S66. doi: 10.1016/j.jcv.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Morshed K. Association between human papillomavirus infection and laryngeal squamous cell carcinoma. J Med Virol. 2010;82(6):1017–1023. doi: 10.1002/jmv.21749. [DOI] [PubMed] [Google Scholar]
- 9.Kim KM, Cho NH, Choi HS, Kim YH, Byeon HK, Min HJ, et al. Effect of human papilloma virus expression on clinical course of laryngeal papilloma. Acta Otolaryngol. 2008;128(10):1138–1144. doi: 10.1080/00016480701827509. [DOI] [PubMed] [Google Scholar]
- 10.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Modern pathology : an official journal of the United States and Canadian Academy of Pathology Inc. 2011. doi:10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed]
- 11.Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24(36):5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 12.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27(12):1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 13.Kumar B, Cordell KG, Lee JS, Prince ME, Tran HH, Wolf GT, et al. Response to therapy and outcomes in oropharyngeal cancer are associated with biomarkers including human papillomavirus, epidermal growth factor receptor, gender, and smoking. Int J Radiat Oncol Biol Phys. 2007;69(2 Suppl):S109–S111. doi: 10.1016/j.ijrobp.2007.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castle PE, Schiffman M, Gravitt PE, Kendall H, Fishman S, Dong H, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002;68(3):417–423. doi: 10.1002/jmv.10220. [DOI] [PubMed] [Google Scholar]
- 16.Schiffman M, Rodriguez AC, Chen Z, Wacholder S, Herrero R, Hildesheim A, et al. A population-based prospective study of carcinogenic human papillomavirus variant lineages, viral persistence, and cervical neoplasia. Cancer Res. 2010;70(8):3159–3169. doi: 10.1158/0008-5472.CAN-09-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens–Part B: biological agents. Lancet Oncol. 2009;10(4):321–322. doi: 10.1016/S1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 18.el Awady MK, Kaplan JB, O’Brien SJ, Burk RD. Molecular analysis of integrated human papillomavirus 16 sequences in the cervical cancer cell line SiHa. Virology. 1987;159(2):389–398. doi: 10.1016/0042-6822(87)90478-8. [DOI] [PubMed] [Google Scholar]
- 19.Braakhuis BJ, Snijders PJ, Keune WJ, Meijer CJ, Ruijter-Schippers HJ, Leemans CR, et al. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst. 2004;96(13):998–1006. doi: 10.1093/jnci/djh183. [DOI] [PubMed] [Google Scholar]
- 20.van Houten VM, Snijders PJ, van den Brekel MW, Kummer JA, Meijer CJ, van Leeuwen B, et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer. 2001;93(2):232–235. doi: 10.1002/ijc.1313. [DOI] [PubMed] [Google Scholar]
- 21.Michiels S, Le Maitre A, Buyse M, Burzykowski T, Maillard E, Bogaerts J, et al. Surrogate endpoints for overall survival in locally advanced head and neck cancer: meta-analyses of individual patient data. Lancet Oncol. 2009;10(4):341–350. doi: 10.1016/S1470-2045(09)70023-3. [DOI] [PubMed] [Google Scholar]
- 22.Greenland S, Robins JM. Identifiability, exchangeability, and epidemiological confounding. Int J Epidemiol. 1986;15(3):413–419. doi: 10.1093/ije/15.3.413. [DOI] [PubMed] [Google Scholar]
- 23.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87(417):178–183. doi: 10.1080/01621459.1992.10475190. [DOI] [Google Scholar]
- 25.Hosmer D, Lemeshow S. Applied survival analysis. New York: Wiley; 1999. [Google Scholar]
- 26.O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48(12):1191–1201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Rose Ragin CC, Taioli E. Second primary head and neck tumor risk in patients with cervical cancer–SEER data analysis. Head Neck. 2008;30(1):58–66. doi: 10.1002/hed.20663. [DOI] [PubMed] [Google Scholar]
- 28.Reddy VM, Cundall-Curry D, Bridger M. Trends in the incidence rates of tonsil and base of tongue cancer in England, 1985–2006. Ann R Coll Surg Engl. 2010;92(8):655–659. doi: 10.1308/003588410X12699663904871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103(9):1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 30.Robinson KL, Macfarlane GJ. Oropharyngeal cancer incidence and mortality in Scotland: are rates still increasing? Oral Oncol. 2003;39(1):31–36. doi: 10.1016/S1368-8375(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 31.Attner P, Du J, Nasman A, Hammarstedt L, Ramqvist T, Lindholm J, et al. The role of human papillomavirus in the increased incidence of base of tongue cancer. Int J Cancer. 2010;126(12):2879–2884. doi: 10.1002/ijc.24994. [DOI] [PubMed] [Google Scholar]
- 32.Nasman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125(2):362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 33.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 34.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110(7):1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 35.Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, Joneberg J, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119(11):2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 36.Klaes R, Woerner SM, Ridder R, Wentzensen N, Duerst M, Schneider A, et al. Detection of high-risk cervical intraepithelial neoplasia and cervical cancer by amplification of transcripts derived from integrated papillomavirus oncogenes. Cancer Res. 1999;59(24):6132–6136. [PubMed] [Google Scholar]
- 37.Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 38.Shi W, Kato H, Perez-Ordonez B, Pintilie M, Huang S, Hui A, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27(36):6213–6221. doi: 10.1200/JCO.2009.23.1670. [DOI] [PubMed] [Google Scholar]
- 39.Bristow RG, Benchimol S, Hill RP. The p53 gene as a modifier of intrinsic radiosensitivity: implications for radiotherapy. Radiother Oncol. 1996;40(3):197–223. doi: 10.1016/0167-8140(96)01806-3. [DOI] [PubMed] [Google Scholar]
- 40.Mellin H, Dahlgren L, Munck-Wikland E, Lindholm J, Rabbani H, Kalantari M, et al. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer. 2002;102(2):152–158. doi: 10.1002/ijc.10669. [DOI] [PubMed] [Google Scholar]
- 41.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 42.Smith EM, Wang D, Kim Y, Rubenstein LM, Lee JH, Haugen TH, et al. P16INK4a expression, human papillomavirus, and survival in head and neck cancer. Oral Oncol. 2008;44(2):133–142. doi: 10.1016/j.oraloncology.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Soria JC, Rodriguez M, Liu DD, Lee JJ, Hong WK, Mao L. Aberrant promoter methylation of multiple genes in bronchial brush samples from former cigarette smokers. Cancer Res. 2002;62(2):351–355. [PubMed] [Google Scholar]
- 44.Fischer CA, Zlobec I, Green E, Probst S, Storck C, Lugli A, et al. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010;126(5):1256–1262. doi: 10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 45.Sethi S, Ali-Fehmi R, Franceschi S, Struijk L, van Doorn LJ, Quint W, et al. Characteristics and survival of head and neck cancer by HPV status: a cancer registry-based study. Int J Cancer. 2012;131(5):1179–1186. doi: 10.1002/ijc.26500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang C, Marsit CJ, McClean MD, Nelson HH, Christensen BC, Haddad RI, et al. Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res. 2012;72(19):5004–5013. doi: 10.1158/0008-5472.CAN-11-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Austin PC. The performance of different propensity score methods for estimating marginal odds ratios. Stat Med. 2007;26(16):3078–3094. doi: 10.1002/sim.2781. [DOI] [PubMed] [Google Scholar]
- 48.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: 10.1002/(SICI)1097-0258(19981015)17:19<2265::AID-SIM918>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 49.Rosenbaum PR. Discussing hidden bias in observational studies. Ann Intern Med. 1991;115(11):901–905. doi: 10.7326/0003-4819-115-11-901. [DOI] [PubMed] [Google Scholar]
- 50.Phaeton R, Wang XG, Einstein MH, Goldberg GL, Casadevall A, Dadachova E. The influence of proteasome inhibitor MG132, external radiation, and unlabeled antibody on the tumor uptake and biodistribution of (188)re-labeled anti-E6 C1P5 antibody in cervical cancer in mice. Cancer. 2010;116(4 Suppl):1067–1074. doi: 10.1002/cncr.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.