Abstract
Primary sinonasal tract and nasopharyngeal adenoid cystic carcinomas (STACC) are uncommon tumors that are frequently misclassified, resulting in inappropriate clinical management. Eighty-six cases of STACC included 45 females and 41 males, aged 12–91 years (mean 54.4 years). Patients presented most frequently with obstructive symptoms (n = 54), followed by epistaxis (n = 23), auditory symptoms (n = 12), nerve symptoms (n = 11), nasal discharge (n = 11), and/or visual symptoms (n = 10), present for a mean of 18.2 months. The tumors involved the nasal cavity alone (n = 25), nasopharynx alone (n = 13), maxillary sinus alone (n = 4), or a combination of the nasal cavity and paranasal sinuses (n = 44), with a mean size of 3.7 cm. Patients presented equally between low and high stage disease: stage I and II (n = 42) or stage III and IV (n = 44) disease. Histologically, the tumors were invasive (bone: n = 66; neural: n = 47; lymphovascular: n = 33), composed of a variety of growth patterns, including cribriform (n = 33), tubular (n = 16), and solid (n = 9), although frequently a combination of these patterns was seen within a single tumor. Pleomorphism was mild with an intermediate N:C ratio in cells containing hyperchromatic nuclei. Reduplicated basement membrane and glycosaminoglycan material was commonly seen. Necrosis (n = 16) and atypical mitotic figures (n = 11) were infrequently present. Pleomorphic adenoma was present in 9 cases; de-differentiation was seen in two patients. Immunohistochemical studies showed positive reactions for pan-cytokeratin, CK7, CK5/6, CAM5.2, and EMA, with myoepithelial reactivity with SMA, p63, calponin, S100 protein and SMMHC. CD117, CEA, GFAP and p16 were variably present. CK20 and HR HPV were negative. STACC needs to be considered in the differential diagnosis of most sinonasal malignancies, particularly poorly differentiated carcinoma, olfactory neuroblastoma and pleomorphic adenoma. Surgery (n = 82), often accompanied by radiation therapy (n = 36), was generally employed. A majority of patients developed a recurrence (n = 52) 2–144 months after initial presentation. Overall mean follow-up was 19.4 years (range 0.4–37.5 years): 46 patients died with disease (mean 6.4 years); 5 were alive with disease (mean 5.4 years), and 35 patients were either alive or had died of unrelated causes (mean 16.3 years). ACC of the SNT is uncommon. Recurrences are common. The following parameters, when present, suggest an increased incidence of either recurrence or dying with disease: mixed site of involvement, high stage disease (stage IV), skull base involvement, tumor recurrence, a solid histology, perineural invasion, bone invasion, and lymphovascular invasion.
Keywords: Adenoid cystic carcinoma, Paranasal sinuses, Nasal cavity, Staging, Prognosis, Histology, Immunohistochemistry
Introduction
Adenocarcinoma of the sinonasal tract (SNT) is separated into salivary gland-type adenocarcinoma and non-salivary gland-type adenocarcinoma [1–4]. Adenocarcinomas of sinonasal tract can originate from the respiratory epithelium or the underlying mucoserous glands, with most (especially salivary gland type) arising from the mucoserous glands [5]. Malignant sinonasal tract neoplasms are relatively uncommon, accounting for between 2 and 3 % of all upper aerodigestive tract malignancies [6]. Squamous cell carcinoma is the most common sinonasal tract cancer, but adenoid cystic carcinoma (ACC) is the second most common malignancy and the most common salivary gland-type tumor of the sinonasal tract (nasal cavity, paranasal sinuses, and nasopharynx, hereinafter referred to collectively as the sinonasal tract, i.e. STACC) [7]. ACCs comprise between 10 and 15 % of all malignant salivary gland tumors. A review of the English literature (1966–2012) reveals over 2,100 cases of STACC, representing approximately 10–18 % of all malignant sinonasal tract neoplasms, and representing approximately 13 % of all ACC of the head and neck (Table 1) [5–119]. STACC is thought to arise from the minor mucoserous glands which lie within the mucosa, below the respiratory-type epithelium of the nasal cavity and paranasal sinuses, although surface derivation has also been postulated [1, 5]. The high frequency of misdiagnosis of this tumor when it arises in the sinonasal tract results in inappropriate therapy and therefore reduced patient outcome. The English literature contains many case reports, small series, and a few larger series, all of which focus on a particular feature. Specifically, the series often present all salivary gland tumor types together [39, 40, 57, 67, 89, 91, 97, 99, 106, 120–127], encompass all head and head anatomic sites, describe adenoid cystic carcinoma only but in all sites [5, 6, 12, 17, 22, 31, 32, 35, 39, 47, 50, 52, 57, 59, 66, 73, 75, 76, 87, 91–93, 98, 100, 101, 106, 112, 113, 117, 128–130], or only one subsite but all tumors combined. Many small series focus on the radiographic findings alone [36, 62, 88, 96, 99, 108–110, 112, 117, 131–134], or specifically address a particular therapy (radiation) [8, 20, 24, 25, 33, 54, 63, 66, 69, 86, 92, 95, 111] for all tumor types of a particular anatomic site. It is the intention of this study to provide a comprehensive analysis of STACC incorporating the use of clinical features, histologic findings, immunohistochemical results, therapies employed, and patient follow-up applied to a group of 86 patients with this tumor, generating statistically significant findings.
Table 1.
| Clinical characteristics | Number n = 2,117 |
|---|---|
| Gender a | |
| Females | 336 |
| Males | 337 |
| Age (in years) | |
| Range | 23–80 |
| Mean | 49.2 |
| Symptom duration (in months) a | |
| Duration (range) | 0.5–96 |
| Duration (mean) | 23.3 |
| Anatomic site a | |
| Nasal cavity alone | 87 |
| Nasopharynx alone | 90 |
| Maxillary sinus alone | 433 |
| Other single sinus | 75 |
| Mixed location (more than one topographic site) | 58 |
| Size (cm) a | |
| Range | 0.5–8 |
| Mean | 3.4 |
aResults are incomplete, as value was not always stated
Methods
One hundred and seven cases of primary sinonasal tract adenoid cystic carcinomas were selected involving the nasal cavity, paranasal sinuses (sphenoid, maxillary, ethmoid, and frontal sinuses) and nasopharynx. The cases were retrieved from the files of the Otorhinolaryngic-Head & Neck Tumor Registry of the Armed Forces Institute of Pathology (AFIP), Washington, DC, between 1970 and 1998 and from the consultation files (2003–2011) of one of the authors (LDRT). However, 21 patients were excluded from further consideration because of at least one of the following reasons: (1) Paraffin blocks were unavailable for additional sections or immunophenotypic analysis; (2) The original submitted case did not have sufficient demographic information supplied from which to obtain adequate follow-up information; and (3) Immunophenotypic analysis confirmed a diagnosis of lymphoma or olfactory neuroblastoma. The cases which were reclassified were all before 1985, and had all been signed out as “poorly differentiated malignant neoplasm,” “consistent with,” “suggestive of,” or “suspicious for” adenoid cystic carcinoma and had not benefited from electron microscopy or immunophenotyping at the time of the original diagnosis. Therefore, the remaining 86 patients compose the subject of this study, chosen from a review of 22,111 (0.33 %) benign or malignant primary sinonasal tract neoplasms seen in consultation during this time. Seventy-one cases were obtained from civilian sources, including university medical centers and foreign contributors, 12 cases from military hospitals, and 3 cases from Veterans Administration Medical Centers. A cohort of these cases was previously reported with a focus on adenoid cystic carcinoma ex pleomorphic adenoma [105], without any of the remaining cases included in other reports.
Materials within the files were supplemented by a review of the patient demographics (gender, age, race) and symptoms at presentation (epistaxis, headache, facial numbness, nerve dysfunction, nasal obstruction, nasal mass, polyps, sinusitis, discharge, difficulty breathing, blurring of vision, pain, hearing changes, otalgia, tinnitus) including duration. In addition, we reviewed the medical history (specifically noting any mention of major salivary gland primary), imaging, surgical pathology and operative reports, and obtained follow-up information by direct written or oral communication with the referring pathologist, patient’s physician, oncology data services and tumor registries, or the patient (patient’s family member[s]). Follow-up data, available for all patients, included information regarding the exact tumor location, the specific treatment modalities used, the presence or absence of recurrent or metastatic disease, and the current status of the disease and patient. The series covers over four decades, with several changes in the staging criteria. This series includes primary tumors from the sinonasal tract, which includes the following sites and subsites: nasal cavity (floor, septum, lateral wall, roof and vestibule); maxillary sinus (floor, anteriolateral, medial and posterior walls, roof); ethmoid sinus, frontal sinus, sphenoid sinus and nasopharynx. The staging system takes into consideration the epicenter of the tumor in these sites and subsites at the time of diagnosis and places them in the context of extension into bone and neighboring sites and structures, including the oral cavity, soft tissues of the cheek and nose, pterygomaxillary fossa, orbit, pituitary, and skull base (including intracranial extension) (Fig. 1) [17, 135]. These determinations were drawn from an analysis of the clinical, imaging and/or operative data. Preoperative imaging studies were available in 47 cases, including computed tomography and magnetic resonance imaging studies. These studies give complementary information about size, exact site of origin and extension into adjacent structures and extent of the tumor. Reports were reviewed in all these cases, with personal review of 31 patients’ images. It is important for the radiologist to carefully assess nerve involvement, especially along the branches of the trigeminal nerve, in order to evaluate possible intracranial extension. Axiomatic, perineural invasion is not unique to adenoid cystic carcinoma [131]. All patients were staged by the authors as part of this report according to the 2010 American Joint Commission on Cancer Staging [136].
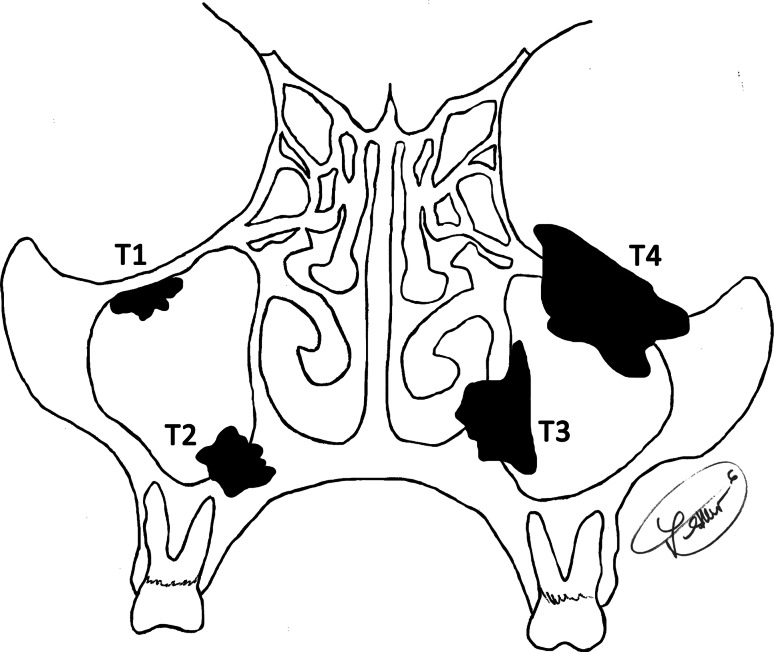
Fig. 1.
A diagrammatic representation of tumor stage, based on a maxillary sinus primary tumor (AJCC 2010). T1: confined to the sinus with no bone destruction; T2: tumor causing bone erosion into hard palate; T3: tumor invades into the subcutaneous tissues (and middle nasal meatus); T4: tumor invades into anterior orbital content
Since most samples were submitted in a fragmented fashion, definitive margins were not assessed. Margins were frequently not identified by the surgeons. Furthermore, as we did not prosect the cases and most were submitted as consultations, assessment of margin status is unreliable. Patients who were found to have a contiguous non-sinonasal site primary ACC (palate, alveolar ridge, orbit) were excluded from further consideration, as were any patients who had a major salivary gland (parotid specifically) primary tumor. No patients in this series were part of a syndrome associated kindred (no familial cancer syndrome). It is important to add that as a tertiary pathology review center, conducting a retrospective review of these patients, we did not treat the patients. This clinical investigation was conducted in accordance and compliance with all statutes, directives, and guidelines of an Internal Review Board authorization (#5968) performed under the direction of Southern California Permanente Medical Group and the Code of Federal Regulations, Title 45, Part 46, and the Department of Defense Directive 3216.2 relating to human subjects in research.
Hematoxylin and eosin-stained slides from all 86 cases were reviewed, with a range of 1–37 slides reviewed per case (mean 4.7 slides), with each slide often containing multiple different sections. The following specific macroscopic and histologic observations were recorded for each tumor: exact tumor location; lateralization; tumor size (greatest dimension in centimeters); surface epithelium (present or absent); surface origin or involvement (Fig. 2); surface ulceration; tumor extension (bone, soft tissue, minor gland, neural or lymphovascular invasion; Fig. 3); architectural patterns of growth [tubular, trabecular, glandular, cribriform, solid, anastomosing cords, cystic spaces, epithelial-myoepithelial carcinoma-like, pleomorphic adenoma (Fig. 4)]; glycosaminoglycan matrix material (Fig. 5); reduplicated basement membrane; necrosis (present or absent); sclerosis; hemorrhage; calcification; nuclear pleomorphism (mild, moderate, severe: Fig. 6); presence of nucleoli; nuclear to cytoplasmic ratio; mitotic figures (number of mitotic figures per 10 high power fields [magnification at ×40 with a ×10 objective lens using an Olympus BX41 microscope]); atypical mitotic figures (present or absent, and defined by abnormal chromosome spread, tripolar or quadripolar forms, circular forms, or indescribably bizarre); and the presence of other microscopic pathologic findings. Neural invasion was defined as invasion of neoplastic cells into the perineural space (perineural) or between nerve fascicles (intraneural), irrespective of the size of the nerve (nerve diameter of ≤1 mm or >1 mm) and whether the nerve was within the tumor or ≤1 cm of the tumor mass [38]. No meaningful separation was achieved by separating perineural from intraneural invasion, and so these groups were combined in this evaluation. Lymphovascular invasion required the presence of neoplastic cells within an endothelial lined space, with or without thrombus and whether free floating or attached to the endothelium. Stromal-epithelail retraction was carefully excluded, seeking endothelial cells before definitive lymphovascular invasion was noted, but without performing immunohistochemistry for a vascular marker. The tumors were misclassified by the contributor in 54.6 % (n = 47), with pleomorphic adenoma, squamous cell carcinoma, basal cell adenoma, monomorphic adenoma, basaloid squamous cell carcinoma, epithelial-myoepithelial carcinoma, inverted papilloma, and carcinoma, not otherwise specified, diagnosed most commonly.
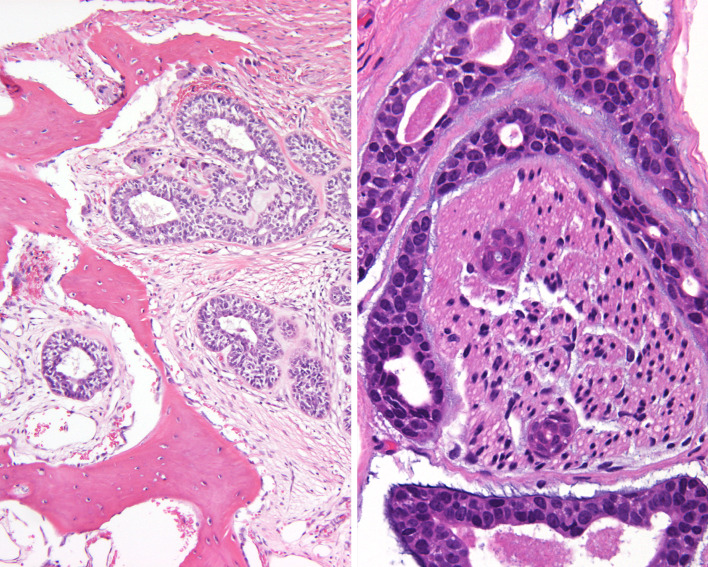
Fig. 2.
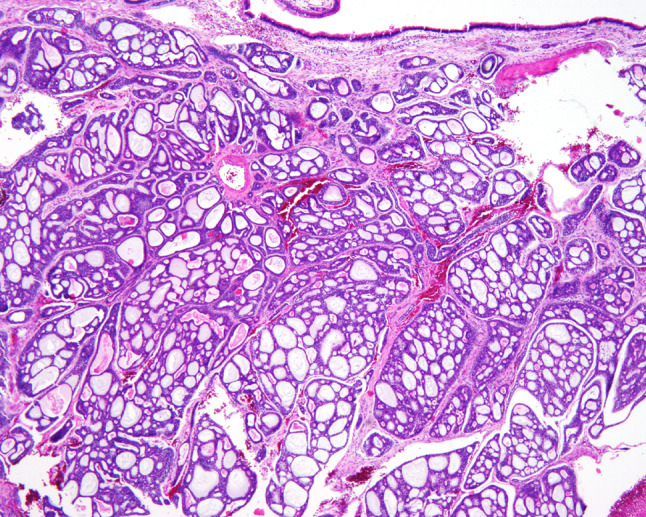
An intact surface mucosa overlying the cribriform and cystic patterns of a sinonasal adenoid cystic carcinoma
Fig. 3.
Bone invasion (left) and perineural and intraneural invasion (right)
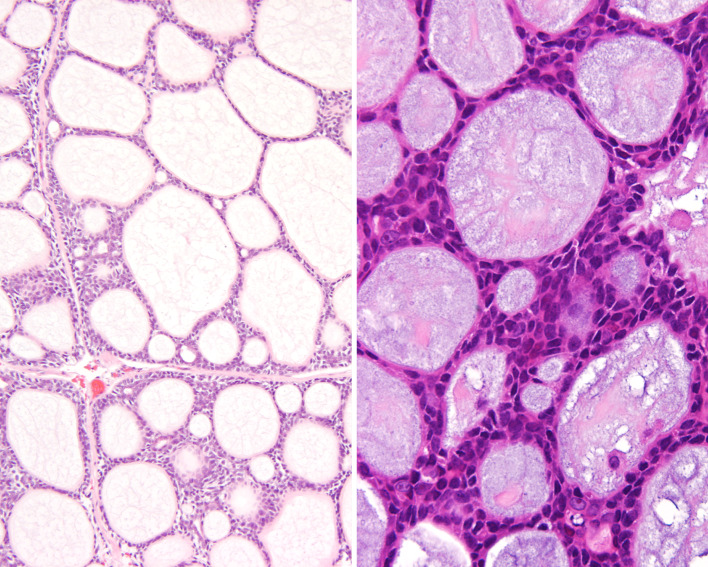
Fig. 4.
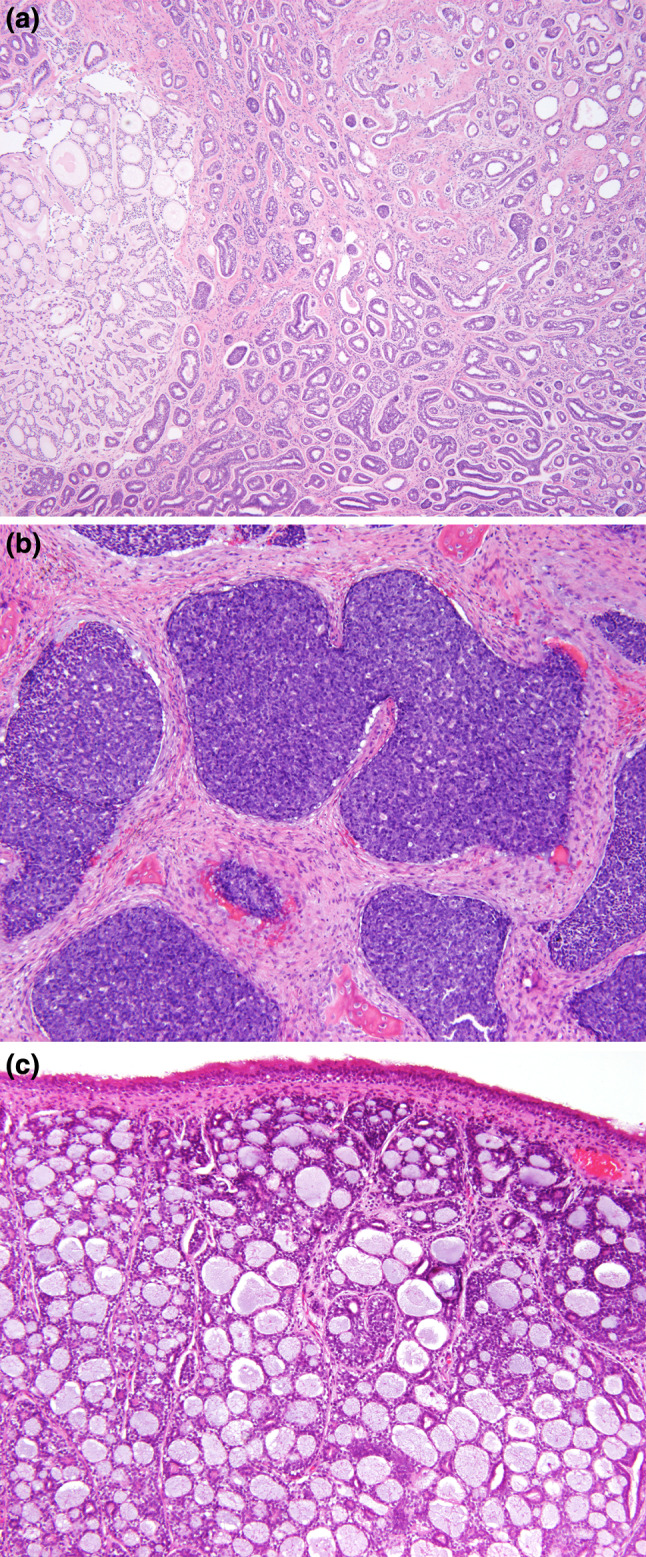
a Low power of a sinonasal adenoid cystic carcinoma showing various patterns of growth. Palisaded neoplastic cells around areas of glycosaminoglycan material. Hyperchromasia of the nuclei is appreciated. b A solid pattern demonstrated focal clefting around the periphery of the nodules of tumor. The cells had a very high nuclear to cytoplasmic ratio, with heavy nuclear chromatin distribution. c The large cystic spaces were filled with light pink to heavily “blue” stained material. There is an intact surface respiratory epithelium
Fig. 5.
“Blue-goo” matrix material could appear eosinophilic and granular (left) while the more characteristically “blue” mucinous material (right) was most common. “Carrot-shaped” nuclei surround the cysts
Fig. 6.
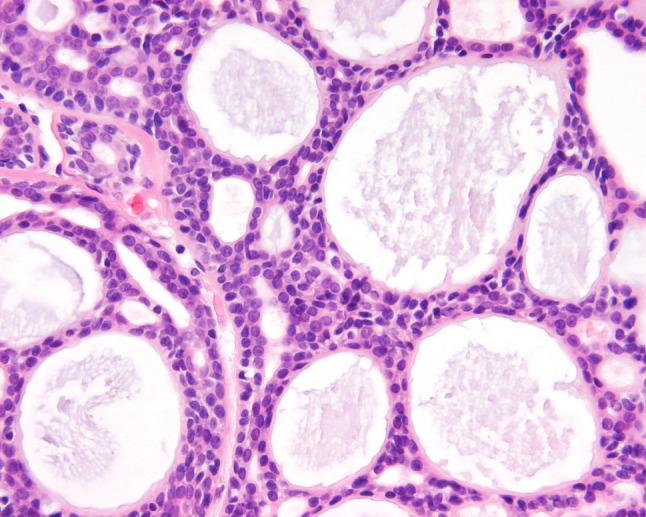
A high power illustrating the bland nuclear appearance and an intermediate to high nuclear to cytoplasmic ratio. Note the small gland or tubule formation, in addition to the larger cysts
Immunophenotypic analysis was performed in all cases with sufficient suitable material by a standardized Envision™ method employing 4 μm-thick, formalin fixed, paraffin embedded sections on a single representative block. Table 2 documents the pertinent, commercially available immunohistochemical antibody panel used. Epitope retrieval was performed, as required by the manufacturer guidelines. Standard positive controls were used throughout, with serum used as the negative control. The antibody reactions were catalogued by location and graded as absent to weak (0–1+), moderate (2+–3+) and strong (4+) staining, and the fraction of positive cells was determined by separating them into four groups: <10 % (focal), 11–50 %, 51–90 %, and >90 % (diffuse); proliferation marker and p53 were separated into <2 %, 2–10, >10 %.
Table 2.
Immunohistochemical panel
| Antigen/antibody | Primary antibody | Company | Dilution | Antigen recovery |
|---|---|---|---|---|
| Cytokeratin-pan (AE1/AE3:M3515) | mm | Dako, Carpinteria, CA | 1:40 | CC1, 30 min |
| CK5/6 (D5/16 B4) | mm | Dako | 1:25 | E2, 20 min |
| CK7 (OV-TL-12/30) | mm | Dako | 1:200 | CC1, 30 min |
| Epithelial membrane antigen (EMA) (E29) | mm | Ventana Medical Systems, Tucson, AZ | Neat | CC1, 30 min |
| CK20 (KS20.8) | mm | Ventana | Neat | CC1, 30 min |
| CEA(p) | rp | Lab Vision, NeoMarkers, Fremont, CA | 1:250 | CC1, 30 min |
| CD117 (C-Kit) | rp | Dako | 1:400 | CC1, 30 min |
| S-100 protein | rp | Dako | 1:2000 | CC1, 30 min |
| Calponin | mm | Abcam, Cambridge, England | Neat | CC1, 30 min |
| p63 (7jul) | mm | Leica Microsystems, Buffalo Grove, IL | 1:40 | E2, 30 min |
| Smooth muscle actin (asm-1) | mm | Leica Microsystems | 1:200 | E2, 20 min |
| Muscle specific actin (HHF35) | mm | Enzo Life Sciences, Farmingdale, NY | 1:100 | CC1, 30 min |
| Smooth muscle myosin heavy chain (SMMS-1) | mm | Dako | 1:100 | CC1, 30 min |
| Glial fibrillary acidic protein (GFAP)(6F2) | mm | Dako | 1:200 | CC1, 30 min |
| p16INK4a (E6H4) | mm | MTM Laboratories (Ventana Medical Systems) | Neat | CC1, 30 min |
| p53 (DO-7) | mm | Dako | Neat | CC1, 30 min |
| Ki67 (MIB1) | mm | Dako | 1:100 | CC1, 30 min |
| MYB (EP769Y) | mm | Epitomics, Inc, Burlingame, CA | 1:200 | CC1, 30 min |
mm Mouse monoclonal, rp rabbit polyclonal
To verify acceptable MYB translocation status and expression as well as human papillomavirus (HPV) status, a small tissue microarray was constructed using two 1 mm cores from 12 random cases with available material. FISH for MYB was performed as follows: Nick translated probes produced from bacterial artificial chromosome (BAC) clones were obtained from Empire Genomics as a hybridization-mixture with human cot-1 DNA. Tissue microarray slides were incubated at 56 °C overnight, deparaffinized using xylene, and rehydrated using a graded series of ethanol baths. Slides were heated in target retrieval solution (Dako) at 95 °C for 40 min, rinsed with distilled water for 5 min, digested with Proteinase K at room temperature for 8 min, rinsed with distilled water for 5 min, then dehydrated with a graded series of alcohol baths. BAC clone RP11-104D9 labeled with 5-Fluorescein and BAC clone RP11-170P19 labeled with 5-carboxy-X-rhodamine (5-Rox) were used. The probe/in situ hybridization buffer mixture was distributed across the microarray area, covered with a glass cover slip, and sealed with rubber cement. Slides were incubated in a humidified chamber at 73 °C for 5 min to denature the microarray DNA followed by 37 °C for 18 h to hybridize with the probes. After hybridization, slides were washed with 2X SSC at room temperature for 5 min, 0.4X SSC/0.3 % NP-40 at 73 °C for 2 min, 2X SSC/0.1 % NP-40 at room temperature for 1 min, and rinsed with distilled water at room temperature for 1 min. Slides were counterstained using DAPI in Vectashield mounting medium (Vector Laboratories), covered with a glass cover slip, and sealed with CytoSeal XYL. Slides were examined on a fluorescence microscope (Imager A.2, Carl Zeiss Imaging Solutions GmbH) equipped with a 150× Plan-APOCHROMA objective and images captured using a digital camera (AxioCam MRm, Zeiss) controlled by AxioVision software (Release 4.8, Zeiss). A minimum of 100 nuclei were examined per specimen to obtain the consensus probe count. MYB immunohistochemistry was performed as follows: Tissue sections were cut and incubated with anti-MYB rabbit monoclonal antibody (clone EP769Y;1:200 dilution; Epitomics Inc., Burlingame, CA, USA) using a DAKO (Carpinteria, CA, USA) autostainer after antigen retrieval for 30 min. The antibody recognizes a synthetic peptide corresponding to residues near the N-terminus of human MYB. The avidin-biotinimmunoperoxidase technique was used. Diaminobenzidine was the chromogen, and sections were counterstained with hematoxylin. MYB immunostaining was considered positive if greater than 5 % of tumor cells displayed strong nuclear immunoreactivity. In situ hybridization for high-risk HPV was performed using an automated benchmark XT system (Ventana Medical Systems, Inc., Tucson, AZ). The INFORM HPV III family 16 probe cocktail, with affinity for high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66), was applied and the reaction was developed using a Hybrid Ready Detection Kit (Ventana Medical Systems, Inc., Tucson, AZ). Positive signals included punctate or diffuse reactivity within tumor nuclei.
A review of publications in English (MEDLINE 1966–2012) was performed, with all cases reported with clinical, histologic, immunophenotypic and/or follow-up information on STACC evaluated and included in the review, but excluding “Quiz” or “Case of the Month” type reports. Many cases were excluded if the lesion arose primarily in the oral cavity, upper lip or oropharynx or represented a different tumor type, or if the information was too generalized and non-specific to make a meaningful interpretation of the demographics, histologic features, immunohistochemistry findings, or patient outcome.
Statistical evaluation was performed using a standard statistics software package with categorical variables analyzed using Chi square tests and Fisher’s Exact tests to compare observed and expected frequency distributions. Comparison of means between groups was made with unpaired t tests or one-way analysis of variance, depending on whether there were two groups or more than two groups, respectively. Multiple comparisons were analyzed using the Tukey method and log-rank analysis. Linear regression was used to investigate two measured variables, and Pearson correlation coefficients were generated to measure the strength of the association. Survival curves were generated using the Kaplan–Meier product-limit method. Confidence intervals of 95 % were generated for all positive findings. The alpha level was set at p < 0.05.
Results
Clinical
The patients included 45 women and 41 men (Table 3) who ranged in age from 12 to 91 years, with a mean age at presentation of 54.4 years (median, 58 years; mode, 60 years). There was no statistical difference in mean age at presentation between the genders (p = 0.401). There was no significant difference in overall survival between the genders (p = 0.687). Of note, there was no significant decrease in overall survival for patients who were ≥60 years of age at initial presentation than those who were younger (p = 0.470).
Table 3.
Clinical characteristics
| Clinical characteristics | Number (n = 86) |
|---|---|
| Gender | |
| Females | 45 (52 %) |
| Males | 41 (48 %) |
| Age (in years) | |
| Range | 12–91 |
| Mean | 54.4 |
| Women (mean) (p = 0.401) | 52.9 |
| Men (mean) | 56.2 |
| Maxillary sinus only (mean) (p = 0.006) | 48.0 |
| Nasal cavity only (mean) | 66.0 |
| Nasopharynx (mean) | 45.0 |
| Mixed locations (mean) | 58.0 |
| Stage I (mean) (p = 0.725) | 52.0 |
| Stage II (mean) | 60.0 |
| Stage III (mean) | 56.0 |
| Stage IV (mean) | 56.0 |
| Symptoms (in months)* | |
| Duration (range) | 0.5–120 |
| Duration (mean) | 18.2 |
| Duration (mean; women) (p = 0.065) | 22.5 |
| Duration (mean; men) | 13.2 |
| Obstructive symptoms (including mass, polyps) | 54 |
| Epistaxis (mean duration: 13.1 mo) (p = 0.200) | 23 |
| Auditory symptoms (hearing changes, otitis, otalgia, tinnitus) | 12 |
| Nerve symptoms (numbness, nerve dysfunction, paralysis, pain) | 11 |
| Nasal discharge, allergies, sinusitis | 11 |
| Visual symptoms (blurred vision, proptosis) | 10 |
| Headache | 7 |
| Neck mass | 1 |
| Anatomic site | |
| Nasal cavity alone | 25 (29 %) |
| Nasopharynx alone | 13 (15 %) |
| Maxillary sinus alone | 4 (5 %) |
| Mixed location (more than one topographic site) | 44 (51 %) |
| Skull base involvement | 26 (30 %) |
| Size (cm) | |
| Range | 1–11 |
| Mean | 3.7 |
| Female (mean) (p = 0.299) | 3.9 |
| Male (mean) | 3.5 |
| In patients with epistaxis (mean) (p = 0.212) | 3.5 |
| Nasopharynx alone (mean) | 2.7 |
| Single anatomic site (mean) (p = 0.0001) | 3.0 |
| Mixed anatomic sites (mean) | 4.4 |
| Carcinoma ex-pleomorphic adenoma | 3.3 |
| I (mean) (p = 0.0001) | 2.7 |
| II (mean) | 3.4 |
| III (mean) | 4.3 |
| IV (mean) | 4.6 |
| Stage | |
| I | 37 (43 %) |
| II | 5 (6 %) |
| III | 15 (17 %) |
| IV | 29 (34 %) |
* More than one symptom may have been experienced by the patients
The patients presented clinically with a variety of symptoms referable to the tumor location where obstructive symptoms (including mass, polyps, cheek swelling; n = 54) and epistaxis (frequent and profuse; n = 23) accounted for the most frequent presenting symptoms. Patients experienced epistaxis for a mean of 13.1 months, a finding which was not statistically significantly related to survival (p = 0.200). Patients frequently reported more than one symptom, including auditory symptoms (changes in hearing, otitis, otalgia tinnitus; n = 12), nerve symptoms (numbness, nerve dysfunction, paresthesia, epiphora, paralysis, anosmia, trismus, pain; n = 11), nasal discharge, allergies, rhinitis or sinusitis (n = 11), visual symptoms (blurred vision, exophthalmos, proptosis, ptosis, diplopia, decreased vision; n = 10), headache (n = 7), or a neck mass (n = 1) or combination thereof.
The duration of symptoms ranged from a couple of weeks to 120 months, with an average of 18.2 months. There was no statistical difference in average length of symptoms between the genders (p = 0.065). We were unable to correlate the clinical presentation with the specific tumor location.
Pathologic Features
Macroscopic
The tumors occurred in the nasal cavity only (n = 25), nasopharynx only (n = 13), maxillary sinus only (n = 4), or in a mixed site (n = 44; nasal cavity, paranasal sinuses, including ethmoid, frontal, sphenoid, and/or nasopharynx) (Table 3; Fig. 1), determined by a combination of imaging studies, intraoperative determination, and gross resection specimen evaluation. Lesions described as “nasal cavity alone” or “nasal cavity and paranasal sinuses” may have had tumors which involved a specific subsite (septum, turbinate) but were not designated as such. None of the tumors in this series involved the oral cavity, lateral orbit (lacrimal gland), or cribriform plate at initial presentation. The tumors ranged in size from 1 to 11 cm, with a mean of 3.7 cm (median, 3.2 cm). There was no statistically significant difference in the size of tumors between the genders (women, 3.9 cm; men, 3.5 cm; p = 0.299). There was a statistical difference in the mean size of tumors which involved certain sites (p = 0.0001), such as nasopharynx alone (mean 2.7 cm), nasal cavity alone (mean 3.0 cm) versus mixed sites (mean 4.4 cm). However, size alone, using both a continuous variable and selected cut-off points (4 cm is shown), did not correlate to a worse patient outcome as an independent parameter, even when carcinoma ex-pleomorphic adenoma cases were excluded (carcinoma ex-pleomorphic adenoma: mean = 3.3 cm; all remaining cases: mean = 3.7 cm) (p = 0.751). The majority of lesions were received as multiple, irregular fragments of soft tissue, especially in the biopsy and wide excision specimens. The cut surface, when not submitted in multiple fragments, was composed of grayish pink to tan firm soft tissue.
Microscopic
The majority of tumors demonstrated, for the most part, overlying respiratory or metaplastic squamous surface epithelium (n = 65) (Figs. 2, 4c; Table 4), with varying degrees of ulceration in most of these cases. Surface involvement and/or derivation of the neoplasm was noted in 12 tumors, although invasion into versus from the surface epithelium was often difficult to accurately determine. Tumor cell invasion into adjacent bone (Fig. 3) was noted in the vast majority of cases (n = 66), and was statistically associated with a worse patient outcome (p = 0.0005). Neural invasion (Fig. 3) was detected in 47 patients. The presence of neural invasion was statistically associated with an adverse patient outcome (p < 0.0001). Lymphovascular invasion was identified in 33 tumors, a finding also associated with a worse patient outcome (p = 0.0058). Single cell infiltration was detected in only a few cases (n = 8).
Table 4.
Microscopic features
| Microscopic characteristic | Number (n = 86) |
|---|---|
| Derivation | |
| Possible surface epithelial involvement | 12 |
| No surface derivation | 74 |
| Neural invasion (p < 0.0001) | 47 |
| Bone invasion (p = 0.0005) | 66 |
| Lymphovascular invasion (p = 0.0058) | 33 |
| Individual, single cell invasion | 8 |
| Growth pattern | |
| Cribriform (≥70 %) | 33 |
| Tubular (≥70 %) | 16 |
| Solid (≥70 %) | 9 |
| Pleomorphism | |
| Mild | 70 |
| Moderate | 14 |
| Severe (de-differentiation) (p = 0.105) | 2 |
| Nuclear to cytoplasmic ratio | |
| Intermediate | 71 |
| High | 15 |
| Sclerosis prominent | 16 |
| Glycosaminoglycan prominent | 13 |
| Reduplicated basement membrane prominent | 52 |
| Necrosis present | 16 |
| Mitotic figures | |
| Present | 71 |
| Mean (per 10 HPF) | 7.3 |
| Range | 0–109 |
| Atypical figures (present) | 11 |
| Other features | |
| Anastomosing cords | 17 |
| Large cystic spaces | 13 |
| Pleomorphic adenoma (carcinoma ex-pleomorphic adenoma) | 9 |
| EMC-like areas | 4 |
| Squamous metaplasia | 3 |
| Dedifferentiation | 2 |
| Calcifications | 2 |
| Margin status | |
| Unknown or positive | 72 |
| Negative (p = 0.103) | 14 |
| Grade | |
| I | 22 |
| II | 37 |
| III | 27 |
HPF high power field
p Values are included to indicate features associated with a poor patient outcome when present
A variable architectural appearance was characteristic both between tumors as well as within tumors, but a specific architectural pattern tended to predominate in each case: cribriform (n = 33; Figs. 2, 4a), tubular (n = 16; Figs. 2, 4a), and solid (n = 9; Fig. 4b), while anastomosing cords or widely dilated cystic spaces (Fig. 4c) were noted more sporadically. In 28 tumors, a 70 % dominant pattern was not detected, and so a specific architectural pattern was not assigned. Tumor cells focally demonstrated a bilayered epithelial-myoepithelial carcinoma-like distribution (n = 4), while two carcinomas had areas of de-differentiation. Nine cases contained benign residual pleomorphic adenoma, in which ACC represented the malignant component of a carcinoma ex-pleomorphic adenoma (see previously reported case series by Toluie et al. [105]). The characteristic glycosaminoglycan matrix material (Fig. 5) was identified in most cases (n = 49), although prominent in 13 cases. This material often created the “rotary telephone dial” appearance. Reduplicated basement membrane material was also noted in most cases, often creating a jigsaw puzzle-like appearance. Rare calcifications were noted. The nuclei displayed a “carrot”, “angular” or “peg” shape as they palisaded around the periphery of the tumor nests, showing only mild to moderate nuclear pleomorphism (Fig. 6). Palisading was often inconspicuous, and so could not be solely relied upon for the diagnosis of ACC. Nucleoli were frequently present, although they were usually small. Irregular, prominent, macronucleoli were only noted rarely. Most cells had a moderate or intermediate nuclear to cytoplasmic ratio, although a high N:C ratio could be found. Mitoses were seen in most cases (n = 71), with a range of 0–109 and a mean of 7.3 mitoses per 10 high power fields (see Materials and Methods). Atypical mitotic figures were only infrequently present (n = 11), and when present were not associated with a worse clinical outcome (p = 0.35). Tumor necrosis, whether apoptosis or comedo-type necrosis (n = 16) was not associated with a worse patient outcome (p = 0.806). Peritumoral fibrosis and inflammation were seen, but were not a dominant feature in any tumor. Intratumoral fibrosis or sclerosis was uncommon (n = 16), but was seen in most of the cases that also contained pleomorphic adenoma. Using standard grading criteria, [137, 138] there were 22, 37 and 27 Grade 1, 2, and 3 tumors, respectively. The 28 tumors which lacked a dominant histologic pattern, were separated based on other grading criteria.
Immunohistochemical Results
All lesions tested reacted with a pan-cytokeratin and CK5/6 (Table 5), the latter sometimes differentially expressed, highlighting the luminal or tubular epithelium. A variety of epithelial markers were tested in this clinical series with CK7 and EMA identified most frequently. CK20 was absent in all cases. The myoepithelial and/or basal zone areas were variably reactive with S100 protein, calponin, smooth muscle actin, and p63 (Fig. 7), while muscle specific actin and glial fibrillary acidic protein was weakly and focally reactive in a few cases (Table 4). CD117 accentuated the epithelial cells, but tended to be found in areas in which a tubular histology predominated (Fig. 7). The proliferation marker (Ki-67) reacted with up to 22 % of the nuclei, but most cases showed reaction in <10 %, with an overall lack of a high proliferation index. p53 was identified in the nuclei of 22 cases, but it was only increased (>10 %) in nine cases. Both of these markers suggest an overall low tumor turnover. p16 was identified in all tested cases (100 %), yielding both a nuclear and cytoplasmic reaction, stronger in the luminal cells, although all cases were negative for high-risk HPV. p16 was noted in both the pleomorphic adenoma and ACC areas, although with a greater intensity in the cytoplasm of the malignant areas, and a stronger nuclear expression in the PA areas.
Table 5.
Immunohistochemical panel results
| Antigen/antibody | Number of cases with positive reactions | Predominant pattern of reactivity |
|---|---|---|
| Pancytokeratin | 42/42 (100 %) | Diffuse, luminal and tubular |
| CK5/6 | 42/42 (100 %) | Diffuse, luminal and tubular |
| CK7 | 42/42 (100 %) | Diffuse, luminal and tubular |
| Epithelial membrane antigen | 40/42 (95 %) | Focal, luminal and tubular |
| CK20 | 0/42 (0 %) | Absent |
| CEA(p) | 28/42 (67 %) | Focal, mostly tubular |
| CD117 | 38/42 (90 %) | Weak to diffuse, luminal |
| S100 protein | 32/42 (76 %) | Diffuse, nuclear and cytoplasmic |
| Calponin | 25/30 (83 %) | Basal layer |
| p63 | 35/42 (83 %) | Diffuse, basal layer |
| Smooth muscle actin | 42/42 (100 %) | Focal to diffuse, abluminal/basal |
| Muscle specific actin | 10/42 (24 %) | Focal, abluminal/basal |
| Smooth muscle myosin heavy chain | 9/13 (69 %) | Focal to diffuse, abluminal/basal |
| Glial fibrillary acidic protein | 4/42 (10 %) | Focal only |
| p16 | 13/13 (100 %) | Focal to diffuse, luminal (nuclear and cytoplasmic) |
| p53 | 31/42 (74 %) | 2–80 % of nuclei positive |
| Ki67 | 32/42 (76 %) | 1–22 % of nuclei positive |
| MYB | 6/11 (55 %) | 5/8 broken by FISH; 4/5 positive for MYB by IHC |
| HR HPV | 0/13 (0 %) | Negative |
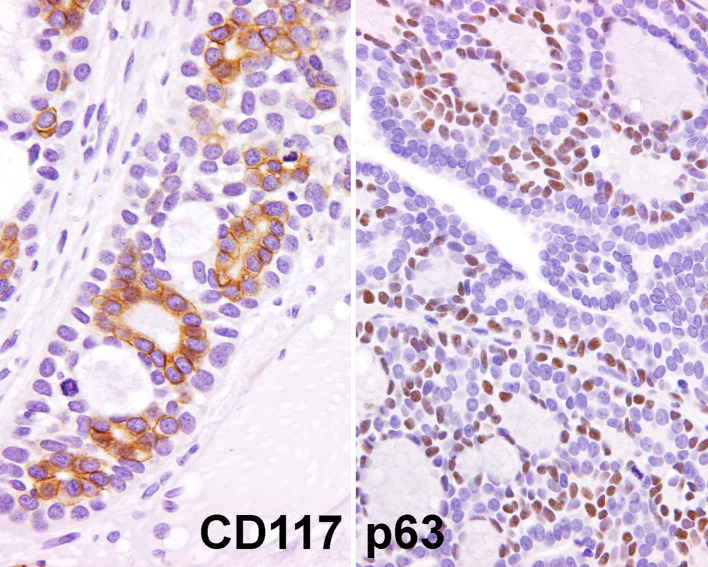
Fig. 7.
Immunohistochemistry was not requisite for the diagnosis. However, the following patterns were present: CD117 (C-kit) was often accentuated around true lumens (left), while the p63 highlighted the basal/myoepithelial cells within the tumor (right)
Of the 12 cases analyzed for MYB translocation using FISH, eight had interpretable results. Of these, five (63 %) showed results consistent with translocation. One of three cases (33 %) negative for the translocation showed positive staining for MYB by immunohistochemistry, whereas 4 of 5 cases with the translocation showed staining. Of cases with non-interpretable FISH results, 2 of 3 showed staining, and one had non-interpretable results.
Treatment and Follow-up
The vast majority of patients were treated by partial or complete surgical excision (n = 82); however, complete extrication of the tumor was not likely due to the complex anatomy of the region. Forty-six patients were treated by surgical resection only without any additional therapy (mean follow-up, 12.2 years): 27 died with disease (mean 6.9 years), 9 had died without evidence of disease (mean 14.1 years), and 10 are alive without evidence of disease at last follow-up (mean 24.9 years). Within this group managed by surgery alone, 30 patients (65 %) developed recurrent disease, and 10 (22 %) developed metastatic disease (lung, bone, liver, brain and skin). Of the patients who developed recurrent disease (managed further by surgery [n = 25] or radiation therapy [n = 5] or a combination with chemotherapy [n = 4]), 25 died with disease (mean 6.7 years), 3 had died without evidence of disease (mean 20.1 years), and 2 are alive without evidence of disease at last follow-up (mean 19.3 years). All 10 patients who developed metastatic disease were dead of their disease (mean 7.4 years). Eight of the 10 patients who had metastatic disease had also developed locally recurrent disease.
An additional 36 patients were managed by an initial combination of surgery and radiation (mean follow-up, 8.8 years): 18 died with disease (mean 5.7 years), 5 had died without evidence of disease (mean 15.4 years), 4 are alive with disease (mean 6.4 years), and 9 are alive without evidence of disease at last follow-up (mean 12.2 years). Within this group managed initially by a combination of surgery and radiation, 22 patients (61 %) developed recurrent disease, and 11 (31 %) developed metastatic disease. Of the patients who developed recurrent disease (managed further by surgery [n = 20] or a combination with radiation and/or chemotherapy [n = 2]), 16 died with disease (mean 6.1 years), 4 are alive with evidence of disease (mean 6.4 years), 2 had died without evidence of disease (18.0 years). Nine of the 11 patients who developed metastatic disease had died of their disease (mean 6.3 years), while 2 patients are alive with disease at last follow-up (mean 8.3 years). Eight of the patients who had metastatic disease had also developed locally recurrent disease.
Four patients were managed with radiation therapy only: 2 patients died without recurrence or metastatic disease (mean 4.1 years), one was alive with disease (1.5 years), and one died with locally recurrent disease at 6.7 years.
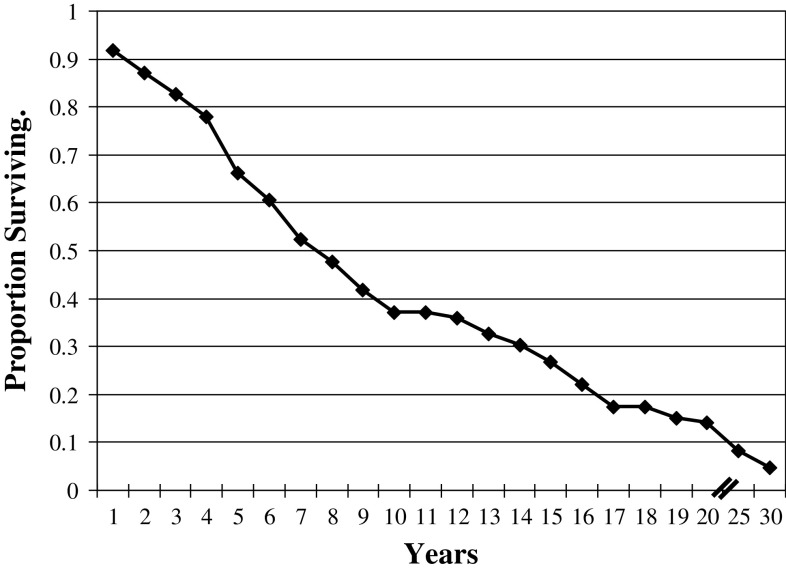
While the overall survival for STACC was grim (53.4 % died with disease, mean of 6.5 years, Fig. 8), a number of patients were alive or had died without evidence of disease at last follow-up (35 patients; 39.5 %; Table 6). Overall, 35 patients were alive or had died without evidence of disease at last contact (mean 16.3 years), while 51 patients were either alive or had died with evidence of disease (mean 6.3 years; Table 6). While a separation was made between patients who had local disease only (n = 18) and those who had disseminated disease (n = 33), there was no statistical difference in overall mean years of survival (mean 6.4 vs. 6.3 years, respectively; p = 0.27). When the patients died with or from their disease (the distinction is often difficult to ascertain retrospectively), they in general died a mean of 6.4 years after initial presentation (Table 6). Twenty-four patients survived for more than 5 years with their tumors before dying of their disease up to 17 years after initial presentation. In general, the 35 patients who were without evidence of disease at last follow-up, had a mean follow-up of 16.3 years. These results yield a raw 5-year survival of 74 % and a raw 10-year survival of 32.9 %. This contrasts to a disease-free 5-year survival of 41.1 % and a disease-free 10-year survival of 24.7 %. The group of forty-three patients who developed recurrent disease had a significantly worse outcome (79 % died with disease, mean: 6.6 years; p < 0.0001) whereas those who did not develop recurrence had significantly fewer deaths from disease (16.7 % died with disease; mean: 5.9 years).
Fig. 8.
Overall actuarial survival of sinonasal tract adenoid cystic carcinoma
Table 6.
Patient outcome based on various parameters (average years of follow-up)
| All patients | A, NED | D, NED | A, D | D, D | Statistical significance | |
|---|---|---|---|---|---|---|
| All patients with follow-up (mean) | 86 (19.4) | 19 (18.9) | 16 (13.3) | 5 (5.4) | 46 (6.4) | n/a |
| Follow-up range (years) | 0.4 to 37.5 | 3.7 to 37.5 | 0.7 to 31.2 | 1.5 to 12.3 | 0.4 to 17.0 | n/a |
| Sex | ||||||
| Males | 41 (9.8) | 8 (16.9) | 10 (12.1) | 2 (8.3) | 21 (6.1) | p = 0.687 |
| Females | 45 (10.9) | 11 (20.3) | 6 (15.2) | 3 (3.6) | 25 (6.7) | |
| Age | ||||||
| <60 years | 47 (12.2) | 12 (22.1) | 5 (18.1) | 4 (5.8) | 26 (7.5) | p = 0.470 |
| ≥60 years | 39 (8.2) | 7 (13.3) | 11 (11.1) | 1 (4.8) | 20 (13.3) | |
| Size | ||||||
| <4.0 cm | 50 (10.6) | 10 (20.0) | 11 (12.3) | 2 (5.5) | 27 (6.8) | p = 0.751 |
| ≥4.0 cm | 36 (10.1) | 9 (17.6) | 5 (15.3) | 3 (5.4) | 19 (5.9) | |
| Lymphovascular invasion | ||||||
| Present | 33 (6.6) | 3 (6.4) | 3 (13.0) | 3 (4.2) | 24 (6.1) | p = 0.0058 |
| Absent | 53 (12.8) | 16 (21.2) | 13 (13.3) | 2 (7.3) | 22 (6.8) | |
| Neural invasion | ||||||
| Present | 47 (6.7) | 6 (7.3) | 2 (15.9) | 4 (6.2) | 35 (6.1) | p < 0.0001 |
| Absent | 39 (14.9) | 13 (24.3) | 14 (12.9) | 1 (2.4) | 11 (7.5) | |
| Bone invasion | ||||||
| Present | 66 (8.8) | 11 (16.7) | 7 (14.8) | 5 (5.5) | 43 (6.2) | p = 0.0005 |
| Absent | 20 (15.7) | 8 (21.9) | 9 (12.1) | n/a | 3 (9.9) | |
| Necrosis | ||||||
| Present | 16 (4.8) | 4 (6.1) | 1 (13.3) | 2 (5.5) | 9 (3.1) | p = 0.806 |
| Absent | 70 (11.7) | 15 (22.3) | 15 (13.3) | 3 (5.4) | 37 (7.2) | |
| Atypical mitoses | ||||||
| Present | 11 (6.2) | 2 (13.5) | 1 (4.9) | 1 (4.3) | 7 (4.6) | p = 0.473 |
| Absent | 75 (11.0) | 17 (19.5) | 15 (13.8) | 4 (5.8) | 39 (6.8) | |
| Histologic type | ||||||
| Cribriform (>70 %) | 33 (11.9) | 6 (24.4) | 10 (11.6) | 1 (12.3) | 16 (7.4) | p = 0.101 |
| Tubular (>70 %) | 16 (11.1) | 7 (13.1) | 2 (9.4) | n/a | 7 (9.7) | |
| Solid (>70 %) | 9 (4.4) | 1 (5.0) | n/a | 1 (2.4) | 7 (4.5) | p = 0.006 |
| Grade | p = 0.089 | |||||
| 1 | 22 (12.5) | 8 (17.6) | 3 (16.1) | n/a | 11 (7.8) | |
| 2 | 37 (11.3) | 6 (28.5) | 8 (9.5) | 2 (6.9) | 21 (7.5) | |
| 3 | 27 (7.5) | 5 (9.4) | 5 (17.6) | 3 (4.5) | 14 (3.8) | |
| Recurrence | ||||||
| With recurrence | 52 (8.2) | 2 (19.3) | 5 (19.3) | 4 (6.4) | 41 (6.5) | p < 0.0001 |
| Without recurrences | 34 (13.7) | 17 (18.5) | 11 (10.5) | 1 (1.5) | 5 (5.92) | |
| Anatomic site | ||||||
| Maxillary sinus alone | 4 (5.8) | n/a | n/a | n/a | 4 (5.8) | p = 0.527 |
| Nasal cavity alone | 25 (15.9) | 8 (24.4) | 8 (14.5) | n/a | 9 (9.7) | p = 0.004 |
| Nasopharynx alone | 13 (8.9) | 3 (14.9) | 2 (7.7) | n/a | 8 (7.0) | p = 0.527 |
| Mixed anatomic sites | 44 (8.1) | 8 (14.9) | 6 (13.5) | 5 (5.5) | 25 (5.2) | p < 0.000 |
| Skull base involvement | 26 (6.5) | n/a | 3 (14.4) | 2 (4.6) | 21 (5.5) | p = 0.005 |
| Stage | ||||||
| I | 37 (13.2) | 9 (23.0) | 10 (13.1) | n/a | 18 (8.3) | p = 0.084 |
| II | 5 (8.8) | 2 (16.4) | n/a | n/a | 3 (3.8) | p = 0.856 |
| III | 15 (8.5) | 2 (19.3) | 4 (13.9) | n/a | 9 (3.6) | p = 0.878 |
| IV | 29 (8.1) | 6 (13.4) | 2 (12.9) | 5 (5.5) | 16 (6.4) | p = 0.013 |
| Treatment | ||||||
| Surgery only | 46 (12.2) | 10 (24.9) | 9 (14.1) | n/a | 27 (6.9) | p = 0.093 |
| Radiation only | 4 (4.1) | n/a | 2 (4.1) | 1 (1.5) | 1 (6.7) | p = 0.277 |
| Combination therapy | 36 (8.8) | 9 (12.2) | 5 (15.4) | 4 (6.4) | 18 (5.7) | p = 0.545 |
Bold values are statistically significant (p < 0.05)
A, NED alive, no evidence of disease; D, NED Dead, no evidence of disease; A WD alive, with disease either local or metastatic; D, WD dead, with disease either local or metastatic; n/a not applicable
Most patients presented with low stage disease (stage I or II; n = 42), while 15 patients presented with stage III tumors and 29 patients presented with stage IV disease (Table 6). Overall, as the stage of disease increased, there was a statistically significant decrease in overall survival and a greater number of patients died from or with disease (Table 6): stage I, 48.6 % (mean 8.3 years); stage II, 60 % (mean 3.8 years); stage III, 60 % (mean 3.6 years); and stage IV, 55.1 % (mean 6.4 years).
As the grade of tumor increased, more patients were likely to die from disease with shorter survival times: Grade 1: 50 % (7.8 years); Grade 2: 57 % (7.5 years); Grade 3: 52 % (3.8 years). Overall, the average follow-up decreased as the grade increased: Grade 1: 12.5 years; Grade 2: 11.3 years; Grade 3: 7.5 years. However, this trend did not quite reach statistical significance (p = 0.089).
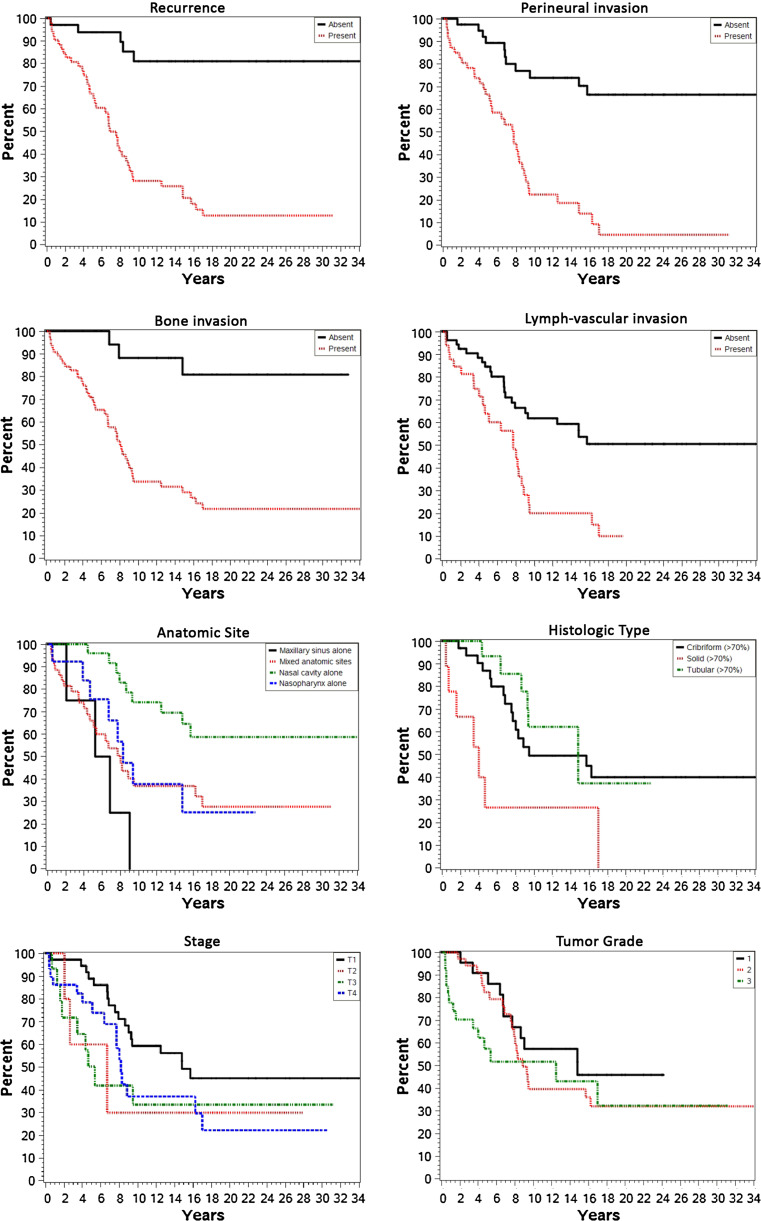
A number of specific clinical and histologic features were evaluated for prognostic significance (Table 6). Patients who had tumors which developed recurrences (p < 0.0001), with perineural invasion (p < 0.0001), bone invasion (p = 0.0005), lymphovascular invasion (p = 0.0058), involved a mixed anatomic site (p = 0.004), arranged in a solid pattern (p = 0.006), or with a high stage at presentation (p = 0.013) were each more likely to experience a worse clinical outcome (Fig. 9).
Fig. 9.
Kaplan-Meier plots based on various statistically significant clinical and histologic parameters. The overall survival of sinonasal tract adenoid cystic carcinoma patients is demonstrated for recurrence, perineural invasion, bone invasion, lymphovascular invasion, epicenter (anatomic site) of the tumor, histologic type, stage and tumor grade
Discussion
Etiology/Embryogenesis
It is believed that STACC arises from the mucoserous glands of the upper aerodigestive tract, although the surface epithelium appeared to be involved in 14 % of cases in this study. While the surface is involved, it does not necessarily imply that the tumor is arising from the surface epithelium [5].
Most recently, it has been well shown that many ACCs, including those outside of the major salivary glands, have a recurrent translation, t(6;9), that juxtaopposes the MYB and NFIB genes [139]. By in situ hybridization, we showed that 62 % of the tumors tested showed a broken MYB gene by FISH. This is within the expected results for ACCs of any site. Some authors have described rare STACCs possibly arising secondary to infection by high-risk HPV, [140, 141] although none of our cases tested harbored the virus.
Clinical Information
In our series as well as the patients reported in the literature, STACC are equally common in men and women. This is similar to salivary gland ACC, although some report a slight female predominance [142]. In general, the mean age for STACC (54.5 years in our series and 49.2 for the literature) is younger than salivary gland ACC. Due to the anatomic location within the sinonasal tract, tumors are able to silently permeate the air-filled spaces, reaching a considerable size before symptoms bring the patient to clinical attention. The presenting symptoms are generally non-specific, mimicking sinusitis or an obstructive lesion, and were present for about 2 years before patients sought clinical attention. This delay in diagnosis contributed to a mean tumor size of about 3.7 cm [6, 86, 106]. The tumors are large for this anatomic site, but it is probably related to slow growth producing symptoms only after the tumors have reached a considerable size. In contrast to salivary gland ACC, pain or other neurological symptoms were not commonly seen in STACC (12.8 %). Most of the tumors involved multiple locations (52 %), although the nasal cavity alone (29 %) was a common finding. Tumor involvement of multiple sites correlated with a worse patient outcome (p < 0.0001) [7, 86]. This is also probably correlated to the majority of patients showing higher stage (III and IV) disease at presentation, involving sensitive structures of the area (optic nerve, brain stem, chiasm). Again, the higher stage disease was associated with a worse outcome (p = 0.013) [6, 7, 24, 86, 106].
Pathology
STACC is characterized by a variety of histologic growth patterns, all of which may be exhibited in a single tumor mass. The three classic histologic patterns of ACC of salivary glands (cribriform, tubular and solid) were seen in STACC, and when these patterns predominated the diagnosis was very straightforward. We perceived two more distinct and unique patterns seen in STACC lesions: anastomosing cords and dilated spaces. Anastomosing cords are characterized by parallel rows of tumor cells which at low power give the appearance of creating pseudocysts. Although the dilated spaces of the cribriform pattern seen in oral cavity ACC may be variable in size, they still are relatively small, producing a “Swiss cheese” appearance. In STACC the cribriform pattern still resembles “Swiss cheese” in areas; however, these spaces may be considerably larger and appear dilated or less structured (flaccid) in other areas of the lesion. These large dilated spaces may be filled with glycosaminoglycan material, reduplicated basement membrane material, or may appear to be empty. The tumor cells in all patterns may be surrounded by a hyalinized material or reduplicated basement membrane. These particular histologic patterns were not seen with sufficient frequency, however, to suggest a difference in outcome. However, the solid histologic pattern (>70 % solid growth) was associated with a worse outcome (p = 0.006). This cut-off is higher than 30 % used by others [6]. However, tumors in the sinonasal tract are fragmented and often in multiple parts, making a calculation of tumor “volume” more difficult. Further, while higher tumor grade was associated with an overall shorter survival (Grade 1: 12.5 years vs. Grade 3: 7.5 years), this difference did not reach statistical significance (p = 0.089), a finding similar to other authors [84]. Interestingly, many of the parameters (necrosis, bone invasion, perineural invasion, dominant pattern) when separately evaluated were significant (see below) while others are not significant (mitoses, atypical mitoses, pleomorphism) or not valid (circumscription) in this anatomic site.
A highly characteristic feature of salivary gland ACC is the tendency to show perineural invasion [6, 142]; however, nerve symptoms were uncommon in STACC (12.8 %). Perineural invasion was not a constant feature in STACC, with perineural or intraneural involvement seen in 54.7 % of cases, within the range of other reported series (40–91 %) [6, 84]. Neural invasion did not correlate to stage, bone invasion or lymphovascular invasion. The diameter of nerve involved, whether intraneural or perineural, and whether the nerves involved are within the tumor or distant from the tumor did not seem to alter the outcome. Therefore, any neural invasion is significant, contributing to a worse patient outcome (p < 0.0001) [6, 35, 38, 143]. It seems that nerve invasion is not related to local invasion or proliferation, and is an independent factor, possibly related to laminin-5 expression [144] or brain-derived neurotrophic factor [60]. Perineural invasion is highly correlated to both local recurrence and poor outcomes (p = 0.0001), and it is well documented that STACC have the highest local recurrence rates of all head and neck sites of ACC [6, 98, 128].
By extension, the presence of both lymphovascular invasion (p = 0.0058) and bone invasion (p = 0.0005) are also statistically significantly correlated to recurrence and a poor patient outcome, similar to other reports [6]. Lymphovascular invasion was seen in about 38 % of cases, with the presence of metastatic disease in distant sites (lung, liver, and bone) a common finding. The erosion of bone by the tumor and expansion into adjacent structures (such as brain stem, skull base, optic chiasm) is probably why this factor is also so closely correlated to recurrence and long term outcome.
Profound pleomorphism, necrosis, and mitoses did not independently predict a worse outcome. The more rapidly dividing the tumor is, the more likely there is to be apoptosis and necrosis. Therefore, it is logical these two factors are related to one another. Atypical mitoses are not common, with only 12.8 % of cases showing atypical mitoses. Further, necrosis is not a common finding in STACC. There is often surface erosion and ulceration, but true tumor necrosis is uncommon (18.6 %).
Immunohistochemical Studies
On a whole, immunohistochemistry was not required to accurately diagnose STACC. The immunohistochemical profile of STACC reveals the presence of two cell populations: epithelial and myoepithelial cells. Keratin and CK5/6 identify the epithelial component, while smooth muscle actin, S100 protein, calponin, smooth muscle myosin heavy chain, and/or p63 identify the basal and myoepithelial cells. CD117 tended to highlight the tubular areas. Although a variety of immunohistochemical stains for both cell types and multiple tumor proliferation markers were performed in our study, they tended not to be specific for STACC (Table 4). The variability in cellular immunoreactivity limits the effectiveness of immunohistochemical panels for definitive diagnosis. However, GFAP is generally negative, and CK20 is always negative. p16 overexpression was seen in all of our cases (luminal nuclear and cytoplasmic reaction; stronger cytoplasmic reaction than in those cases that had associated pleomorphic adenoma), but does not necessarily imply biologically integrated HPV as a potential etiology, as it does with oropharyngeal carcinoma [12]. None of the cases tested contained high-risk HPV (which includes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66) by in situ hybridization, different from a recent series reporting the presence of HPV 33 specifically [141].
Ki-67 ranged from non-reactive up to 22 %. Using a cutoff of 5 % or higher, there was no statistically significant difference in outcome for those with a high proliferation index versus those with a low proliferation index: mean 8.2 versus 10.5 years, respectively; and 64.3 versus 53.6 % dead of disease, respectively. This is different from others who have reported a statistically significant difference [76, 77, 105]. However, an increased ki-67 index is noted in pleomorphic adenoma that undergo malignant transformation to ACC ex-pleomorphic adenoma [105, 145, 146].
Differential Diagnosis
STACC may morphologically masquerade as a variety of benign and malignant neoplasms. In this series, 54.6 % of cases were misclassified at the time of contribution. In general, the differential diagnosis of STACC includes basaloid squamous cell carcinoma (BSCC), olfactory neuroblastoma, small cell neuroendocrine carcinoma, and epitheial-myoepthelial carcinoma, with other tumors much less frequently observed. The short term biologic behavior of ACC is much less aggressive than that of BSCC with raw 5 year survival rates of about 75 %. Regional lymph node metastasis is infrequent at presentation in STACC and distant metastasis occurs late in the disease process. Histologically, ACC and BSCC may exhibit similar growth patterns, since both can show reduplicated basement membrane material and intercellular mucohyaline material. Squamous cell differentiation is lacking in ACC and the cells tend to be smaller and more uniform, with angulated nuclei. Immunohistochemical stains may assist in differentiating ACC from BSCC, but there is considerable overlap so as to make them marginally useful [147]. Olfactory neuroblastoma (ONB) is a malignant neoplasm thought to arise from the olfactory membrane of the sinonasal tract in the ethmoid sinus (cribriform plate). High grade ONB may present diagnostic difficulties with solid variant STACC. However, high grade ONB grows in a lobular pattern, made up of pleomorphic cells with enlarged, hyperchromatic nuclei, variable nucleoli, increased mitotic activity and necrosis (confluent areas or individual cell). True neural rosettes (Flexner-Wintersteiner type) may be present. Peripheral nuclear palisading is seen in STACC and not in ONB. Additionally, the immunohistochemical profile of ONB shows little to absent cytokeratin, p63, and CD99 reactivity, while showing reactivity with chromogranin, synaptophysin, and CD56, with S100 protein highlighting a peripheral sustentacular cell pattern [148]. Sinonasal undifferentiated carcinoma (SNUC) is a highly aggressive malignant neoplasm of the nasal cavity and the paranasal sinuses with absent histologic differentiation. The cellular pleomorphism, hyperchromasia, scant cytoplasm, prominent nucleoli, increased mitotic activity with atypical forms, and usually well developed necrosis are different from STACC. Further, the cribriform and tubular growth patterns, presence of reduplicated basement membrane-like material, and glycosaminoglycan material are absent in SNUC. SNUC are consistently reactive with epithelial markers, but CD117 and p63 are usually absent. Adenosquamous carcinoma (ASC) has an admixture of squamous cell carcinoma and adenocarcinoma, features absent in STACC. The surface epithelium with dysplasia, in situ carcinoma or various proportions of squamous differentiation in addition to peripheral nuclear palisading are lacking in STACC [149]. Neuroendocrine carcinoma (small cell or large cell types) tend to be more destructive clinically, with cells showing a high nuclear to cytoplasmic ratio, salt-and-pepper nuclear chromatin distribution, high mitotic rate and tumor necrosis. There is no glandular or cribriform pattern, although a rosette pattern could mimic a cribriform appearance. The usually well developed dot-like to punctate keratin immunoreactivity and strong, diffuse synaptophysin, chromogranin, CD56, and NSE reactivity should help to differentiation between STACC and neuroendocrine carcinoma. Importantly, CD117 is positive in both tumors, while CK7 is negative in neuroendocrine carcinomas and strongly positive in most STACC [29, 108, 150]. Epithelial-myoepithelial carcinoma usually has a well defined biphasic appearance, with a dual population. The myoepithelial cells often have a cleared cytoplasm. This tumor type does not show a cribriform pattern. An EMC-like pattern can be seen in adenoid cystic carcinoma. Generally, sufficient sampling will help make this separation. Finally, it is important to consider that ACC may be the malignant component of a carcinoma ex-pleomorphic adenoma, as noted in nine of the current cases. However, it is probably more important to thoroughly sample any SNT pleomorphic adenoma to exclude an ACC, than to necessarily try to identify a pleomorphic adenoma in a patient presenting with ACC [23, 36, 105].
Treatment
Patients included in this report were managed at more than 80 different hospitals by a team of surgeons, oncologists, and radiation therapists, among others. While the diagnosis was rendered during the time of initial evaluation and treatment (i.e., team knew the diagnosis during initial treatment rather than finding out the correct diagnosis at the time of a recurrence), inexperience with this uncommon tumor will result in differences in surgical techniques employed or follow-on chemoradiation protocols implemented. It is for these reasons that specific recommendations about surgical technique, radiation protocols or doses of chemotherapy regimens cannot be reliably postulated.
Patients were managed by surgery alone or surgery combined with radiation therapy. It is nearly impossible to achieve complete microscopical resection of STACC. Even when surgical margins may be negative, skip lesions along peripheral nerve trunks invalidate a negative margin. In this study, as in many others [7, 52, 84, 86, 151], determination of margins could not be reliably performed, but perhaps apropos if the data is dearth. Perhaps, therefore, prudence would suggest discontinuing or greatly reducing the submission of multiple frozen section margins which unnecessarily prolong surgery, introduce frozen section artifacts, and provide a false sense of security to the clinician and patient about the success of extirpation. Knowing the morbidity of COMMANDO-type (COMbined MAxilectomy and Neck Dissection Operation) procedures in a disease where surgery offers at best palliative care over the long term, the surgical approach can be tempered to preserve functionality and a better cosmetic outcome. It seems that no matter what approach is employed, ultimately if followed long enough the disease seems to recur. Further, with STACC showing such a high proclivity for perineural invasion, extension into the skull base is quite readily observed (30 %). Because the tumor is slow growing and often has skip lesions, skull base disease is often a late finding. As the skull base has a limited blood supply, chemotherapy is not effective in this setting, although radiation may be palliative [6, 52, 84, 86, 152]. When the skull base was affected, overall survival is much shorter and more patients died with disease (p = 0.005): 26 patient had skull base involvement, surviving an average of 6.5 years and 80.8 % dead of disease versus patients without skull base involvement having an average survival of 12.1 years and 41.7 % dead of disease. These findings are similar to other reviews [6, 52, 84].
The majority of patients were managed by surgery alone (53.4 %), but the remaining patients received combination surgery and radiation therapy. Due to the prolonged clinical course, radiation when combined with surgery does not seem to significantly alter overall patient outcome (p = 0.545), but may result in longer disease free survival. One of the factors may be that the doses required for local control in ACC (>70 Gy) often cannot be achieved in the confines of the SNT without violating tolerance doses for the adjacent organs or structures (ocular or visual toxic effects: keratitis, photophobia, conjunctivitis, blindness; neurologic: seizures, short-term memory loss, brain changes) [6, 55, 86, 92]. In this series, patients managed with surgery alone, had an average follow-up of 12.2 years, with 58.7 % dead of disease, while patients managed with combination therapy had an average follow-up of 8.8 years, with 50 % dead of disease. It is possible that tumors managed by surgery alone were more localized and thus prone to a better prognosis without any additional therapy. Since the disease is progressive and indolent, radiation is probably at best palliative, delaying rather than preventing recurrences and providing symptomatic relief while not significantly altering long term outcome [7, 106]. Modifications of radiation regimens (carbon ion boost and photo intensity-modulated radiation therapy) may yield a better response, but requires more careful evaluation [24, 86, 92]. While STACC is radiosensitive, radiation alone is not considered curative [6]. Chemotherapy does not seem to significantly impact outcome or have a role in the management of STACC [69].
Prognosis
The overall 5-year and ten-year survival rate were 67.4 and 37.2 %, respectively, while the 5-and 10-year disease-free survival rate were 45.3 and 36.0 %, respectively. These findings are similar to major salivary gland survival which is 76.9 and 61.6 % for 5- and 10 year overall survival rate, and 44.2 and 23.0 % for 5- and 10-year disease free survival rates, respectively [7, 10, 11, 56, 59, 142]. Patients may, therefore, live with local disease and/or metastatic disease for extended periods of time, indicating the progressive and indolent nature of STACC, suggesting the 10-year survival rate may be more important and further underscoring the requirement for long-term clinical follow-up and management. The overall mortality rate was 53 % (mean 6.5 years). The overall survival for STACC of 67.4 % at 5-years is better than for age- and stage-matched patients with sinonasal tract squamous cell carcinoma with 34–57 % alive at 5-years [11, 44, 45]. However, for STACC, the prolonged nature of the disease suggests that the histologic type and not just the stage must be taken into consideration when planning management and follow-up protocols.
The majority of patients in this series developed recurrence (60.5 %), within the range of other reported studies (36–56 %) [6, 84]. It is important to note that recurrences often developed soon after resection, but late recurrences can be seen up to 14.8 years after initial presentation. When distant metastases are present, lung, liver, and bone are the most common sites, followed by brain, kidney, skin and soft tissues [10, 57, 65, 76, 99, 121, 153].
The vast majority of patients present with local–regional disease only (i.e., no lymph node or distant metastasis), a finding confirmed in other studies [6, 84], although local disease does not imply low stage disease. In fact, the majority of patients present with high stage disease (stage III and IV, 51.1 %), a finding associated with a worse prognosis (p = 0.013). This finding also correlates with mixed site location (i.e., more than one anatomic site involved), along with involvement of the skull base (p = 0.005). Patients with tumors of the nasal cavity alone had a better prognosis than patients with tumors in the nasopharynx alone or patients with mixed anatomic sites (p < 0.0001). Skull base involvement was also a statistically significant negative prognostic finding (p = 0.005) [6]. With the known propensity of ACC to involve nerves, perineural involvement in the sinonasal tract region correlates with more advanced tumors and higher stage disease, with the attendant proximity to vital structures, limiting the ability to cure the patient [99]. Further, only 38.4 % of patients developed metastatic disease. This may be due in part to the periostium and bone acting as a barrier that is not seen in major salivary gland primaries, and the relatively sparse lymphatics within the bones of the sinonasal tract in comparison to major salivary gland sites [100].
Neural, bone, and lymphovascular invasion were all associated with a statistically significant difference in patient outcome (p < 0.0001, p = 0.0005, p = 0.0058, respectively). Poor prognosis was also associated with a mixed anatomic location (p < 0.0001), a high stage at presentation (stage IV, p < 0.013), skull base involvement (p = 0.005), recurrence (p < 0.0001), and a predominantly (>70 %) solid histology (p = 0.006). Although with variable results, these findings are supported by others who have studied individual factors [45].
Conclusion
In conclusion, STACC, while rare, are distinct mucosal tumors that represent the most common adenocarcinoma of the sinonasal tract. The tumors display a variety of histologic patterns, including: cribriform, tubular, solid, anastomosing cords, and dilated spaces. All of these patterns may be seen in an individual tumor. The diagnosis is substantially made on an H&E stained slide, with immunohistochemistry occasionally helpful in separation from tumors in the differential diagnosis. In general, the prognosis of STACC is better than other malignancies of the sinonasal tract. Poor prognosis is statistically significantly associated with high tumor stage (p = 0.013), skull base involvement (p = 0.005), lymphovascular invasion (p = 0.0058), solid histology (p = 0.006), bone invasion (p = 0.0005), perineural invasion (p < 0.0001), a mixed anatomic site (p < 0.0001), and tumor recurrence (p < 0.0001).
Acknowledgments
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of Southern California Permanente Medical Group. A special thanks to Ms. Hannah Herrera for her research assistance.
References
- 1.Eveson JW. Salivary gland-type carcinomas. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics: head and neck tumours. Lyon: IARC Press; 2005. pp. 24–25. [Google Scholar]
- 2.Franchi A, Santucci M, Wenig BM. Adenocarcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics head and neck tumours. Lyon: IARC Press; 2005. pp. 20–23. [Google Scholar]
- 3.Kleinsasser O, Schroeder HG. Adenocarcinomas of the inner nose after exposure to wood dust. Morphological findings and relationships between histopathology and clinical behavior in 79 cases. Arch Otorhinolaryngol. 1988;245:1–15. doi: 10.1007/BF00463541. [DOI] [PubMed] [Google Scholar]
- 4.Barnes L. Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Am J Surg Pathol. 1986;10:192–202. doi: 10.1097/00000478-198603000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gnepp DR, Heffner DK. Mucosal origin of sinonasal tract adenomatous neoplasms. Mod Pathol. 1989;2:365–371. [PubMed] [Google Scholar]
- 6.Lupinetti AD, Roberts DB, Williams MD, Kupferman ME, Rosenthal DI, Demonte F, et al. Sinonasal adenoid cystic carcinoma: the M. D. Anderson Cancer Center experience. Cancer. 2007;110:2726–2731. doi: 10.1002/cncr.23096. [DOI] [PubMed] [Google Scholar]
- 7.Rhee CS, Won TB, Lee CH, Min YG, Sung MW, Kim KH, et al. Adenoid cystic carcinoma of the sinonasal tract: treatment results. Laryngoscope. 2006;116:982–986. doi: 10.1097/01.mlg.0000216900.03188.48. [DOI] [PubMed] [Google Scholar]
- 8.Ang KK, Jiang GL, Frankenthaler RA, Kaanders JH, Garden AS, Delclos L, et al. Carcinomas of the nasal cavity. Radiother Oncol. 1992;24:163–168. doi: 10.1016/0167-8140(92)90375-5. [DOI] [PubMed] [Google Scholar]
- 9.Benazzou S, Arkha Y, Boulaadas M, Derraz S, Essakali L, Kzadri M. Nasal adenoid cystic carcinoma with intracranial extension. J Craniofac Surg. 2006;17:1026–1029. doi: 10.1097/01.scs.0000234984.99863.de. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharyya N. Factors predicting survival for cancer of the ethmoid sinus. Am J Rhinol. 2002;16:281–286. [PubMed] [Google Scholar]
- 11.Bhattacharyya N. Survival and staging characteristics for non-squamous cell malignancies of the maxillary sinus. Arch Otolaryngol Head Neck Surg. 2003;129:334–337. doi: 10.1001/archotol.129.3.334. [DOI] [PubMed] [Google Scholar]
- 12.Boland JM, McPhail ED, Garcia JJ, Lewis JE, Schembri-Wismayer DJ. Detection of human papilloma virus and p16 expression in high-grade adenoid cystic carcinoma of the head and neck. Mod Pathol. 2012;25(4):529–36. doi: 10.1038/modpathol.2011.186. [DOI] [PubMed] [Google Scholar]
- 13.Bridger MW, Beale FA, Bryce DP. Carcinom of the paranasal sinuses–a review of 158 cases. J Otolaryngol. 1978;7:379–388. [PubMed] [Google Scholar]
- 14.Buffoli A, Olivetti L, Micheletti E, Facchetti F, Moretti R. Degree of malignancy of maxillary sinus cylindromas in relation to histologic characteristics. Tumori. 1982;68:127–131. doi: 10.1177/030089168206800205. [DOI] [PubMed] [Google Scholar]
- 15.Cantu G, Solero CL, Mariani L, Salvatori P, Mattavelli F, Pizzi N, et al. Anterior craniofacial resection for malignant ethmoid tumors–a series of 91 patients. Head Neck. 1999;21:185–191. doi: 10.1002/(sici)1097-0347(199905)21:3<185::aid-hed1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Cantu G, Bimbi G, Miceli R, Mariani L, Colombo S, Riccio S, et al. Lymph node metastases in malignant tumors of the paranasal sinuses: prognostic value and treatment. Arch Otolaryngol Head Neck Surg. 2008;134:170–177. doi: 10.1001/archoto.2007.30. [DOI] [PubMed] [Google Scholar]
- 17.Carinci F, Curioni C, Padula E, Calearo C. Cancer of the nasal cavity and paranasal sinuses: a new staging system. Int J Oral Maxillofac Surg. 1996;25:34–39. doi: 10.1016/s0901-5027(96)80009-9. [DOI] [PubMed] [Google Scholar]
- 18.Carrau RL, Petruzzelli G, Cass SP. Adenoid cystic carcinoma of the nasopharynx. Otolaryngol Head Neck Surg. 1995;112:501–502. doi: 10.1016/S0194-59989570295-4. [DOI] [PubMed] [Google Scholar]
- 19.Caruso VG, Roncace EA, Brennan MT. Cylindroma of the sphenoid sinus. A study of two cases. Trans Pa Acad Ophthalmol Otolaryngol. 1973;26:32–35. [PubMed] [Google Scholar]
- 20.Chen AM, Daly ME, Bucci MK, Xia P, Akazawa C, Quivey JM, et al. Carcinomas of the paranasal sinuses and nasal cavity treated with radiotherapy at a single institution over five decades: are we making improvement? Int J Radiat Oncol Biol Phys. 2007;69:141–147. doi: 10.1016/j.ijrobp.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Chen AY, Stewart MG. Pathologic quiz case 1. Adenoid cystic carcinoma (ACC) of the ethmoid sinus. Arch Otolaryngol Head Neck Surg. 1995;121:1320–3. [PubMed] [Google Scholar]
- 22.Chilla R, Schroth R, Eysholdt U, Droese M. Adenoid cystic carcinoma of the head and neck. Controllable and uncontrollable factors in treatment and prognosis. ORL J Otorhinolaryngol Relat Spec. 1980;42:346–367. doi: 10.1159/000275515. [DOI] [PubMed] [Google Scholar]
- 23.Cimino-Mathews A, Lin BM, Chang SS, Boahene KD, Bishop JA. Carcinoma ex pleomorphic adenoma of the nasal cavity. Head Neck Pathol. 2011;5:405–409. doi: 10.1007/s12105-011-0262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Combs SE, Konkel S, Schulz-Ertner D, Munter MW, Debus J, Huber PE, et al. Intensity modulated radiotherapy (IMRT) in patients with carcinomas of the paranasal sinuses: clinical benefit for complex shaped target volumes. Radiat Oncol. 2006;1:23. doi: 10.1186/1748-717X-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daly ME, Chen AM, Bucci MK, El-Sayed I, Xia P, Kaplan MJ, et al. Intensity-modulated radiation therapy for malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys. 2007;67:151–157. doi: 10.1016/j.ijrobp.2006.07.1389. [DOI] [PubMed] [Google Scholar]
- 26.David SS. Adenoid cystic carcinoma of the maxillary sinus. J Indian Med Assoc. 1976;66:15–17. [PubMed] [Google Scholar]
- 27.Deb T, Thingbaijam S, Devi HP, Singh MM, Singh TH, Sanasam JC. Sinonasal and nasopharyngeal adenoidcystic carcinoma: report of four cases. J Indian Med Assoc. 2004;102(102):104. [PubMed] [Google Scholar]
- 28.Delbouck C, Roper N, Aubert C, Souchay C, Choufani G, Hassid S. Unusual presentation of adenoid cystic carcinoma of the maxillary antrum. B-ENT. 2009;5:265–268. [PubMed] [Google Scholar]
- 29.Demonte F, Ginsberg LE, Clayman GL. Primary malignant tumors of the sphenoidal sinus. Neurosurgery. 2000;46:1084–1091. doi: 10.1097/00006123-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Dickhoff P, Wallace CJ, MacRae ME, Campbell WN. Adenoid cystic carcinoma: an unusual sellar mass. Can Assoc Radiol J. 1993;44:393–395. [PubMed] [Google Scholar]
- 31.Dolan EJ, Schwartz ML, Lewis AJ, Kassel EE, Cooper PW. Adenoid cystic carcinoma: an unusual neurosurgical entity. Can J Neurol Sci. 1985;12:65–68. doi: 10.1017/s0317167100046618. [DOI] [PubMed] [Google Scholar]
- 32.Douglas JG, Laramore GE, ustin-Seymour M, Koh WJ, Lindsley KL, Cho P, et al. Neutron radiotherapy for adenoid cystic carcinoma of minor salivary glands. Int J Radiat Oncol Biol Phys. 1996;36:87–93. doi: 10.1016/s0360-3016(96)00213-1. [DOI] [PubMed] [Google Scholar]
- 33.Ellis ER, Million RR, Mendenhall WM, Parsons JT, Cassisi NJ. The use of radiation therapy in the management of minor salivary gland tumors. Int J Radiat Oncol Biol Phys. 1988;15:613–617. doi: 10.1016/0360-3016(88)90302-1. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson MJ, Dewar JA. Locally recurrent adenoid cystic carcinoma of the left antrum: response to epirubicin, cisplatin and 5-fluorouracil. Clin Oncol (R Coll Radiol) 2001;13:236–237. [PubMed] [Google Scholar]
- 35.Fordice J, Kershaw C, El Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125:149–152. doi: 10.1001/archotol.125.2.149. [DOI] [PubMed] [Google Scholar]
- 36.Freeman SR, Sloan P. Carcinoma ex-pleomorphic adenoma of the nasal septum with adenoid cystic and squamous carcinomatous differentiation. Rhinology. 2003;41:118–121. [PubMed] [Google Scholar]
- 37.Fukuda S, Sakai N, Kamata SE, Nameki H, Kishimoto S, Nishikawa N, et al. Surgical results of skull base surgery for the treatment of head and neck malignancies involving skull base: multi-institutional studies on 143 cases in Japan. Auris Nasus Larynx. 2001;28(Suppl):S71–5. doi: 10.1016/s0385-8146(01)00083-9. [DOI] [PubMed] [Google Scholar]
- 38.Gil Z, Carlson DL, Gupta A, Lee N, Hoppe B, Shah JP, et al. Patterns and incidence of neural invasion in patients with cancers of the paranasal sinuses. Arch Otolaryngol Head Neck Surg. 2009;135:173–179. doi: 10.1001/archoto.2008.525. [DOI] [PubMed] [Google Scholar]
- 39.Goepfert H, Luna MA, Lindberg RD, White AK. Malignant salivary gland tumors of the paranasal sinuses and nasal cavity. Arch Otolaryngol. 1983;109:662–668. doi: 10.1001/archotol.1983.00800240028005. [DOI] [PubMed] [Google Scholar]
- 40.Gotte K, Ganssmann S, Affolter A, Schafer C, Riedel F, Arens N, et al. Dual FISH analysis of benign and malignant tumors of the salivary glands and paranasal sinuses. Oncol Rep. 2005;14:1103–1107. [PubMed] [Google Scholar]
- 41.Greenbaum EI, Gunn W, Rappaport I, O’Loughlin BJ. Cylindroma of the maxillary antrum. A case presentation and review of the literature. Radiol Clin Biol. 1970;39:419–426. [PubMed] [Google Scholar]
- 42.Gullane PJ, Conley J. Carcinoma of the maxillary sinus. A correlation of the clinical course with orbital involvement, pterygoid erosion or pterygopalatine invasion and cervical metastases. J Otolaryngol. 1983;12:141–145. [PubMed] [Google Scholar]
- 43.Hara HJ. Cancer of the nasopharynx. Review of the literature. Report of 72 cases. Laryngoscope. 1969;79:1315–1329. doi: 10.1288/00005537-196907000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Haraguchi H, Ebihara S, Saikawa M, Mashima K, Haneda T, Hirano K. Malignant tumors of the nasal cavity: review of a 60-case series. Jpn J Clin Oncol. 1995;25:188–194. [PubMed] [Google Scholar]
- 45.Harbo G, Grau C, Bundgaard T, Overgaard M, Elbrond O, Sogaard H, et al. Cancer of the nasal cavity and paranasal sinuses. A clinico-pathological study of 277 patients. Acta Oncol. 1997;36:45–50. doi: 10.3109/02841869709100731. [DOI] [PubMed] [Google Scholar]
- 46.Hawkins RB, Wynstra JH, Pilepich MV, Fields JN. Carcinoma of the nasal cavity–results of primary and adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 1988;15:1129–1133. doi: 10.1016/0360-3016(88)90194-0. [DOI] [PubMed] [Google Scholar]
- 47.Higashi K, Jin Y, Johansson M, Heim S, Mandahl N, Biorklund A, et al. Rearrangement of 9p13 as the primary chromosomal aberration in adenoid cystic carcinoma of the respiratory tract. Genes Chromosomes Cancer. 1991;3:21–23. doi: 10.1002/gcc.2870030105. [DOI] [PubMed] [Google Scholar]
- 48.Howard DJ, Lund VJ. Reflections on the management of adenoid cystic carcinoma of the nasal cavity and paranasal sinuses. Otolaryngol Head Neck Surg. 1985;93:338–341. doi: 10.1177/019459988509300309. [DOI] [PubMed] [Google Scholar]
- 49.Howard DJ, Lund VJ. The role of midfacial degloving in modern rhinological practice. J Laryngol Otol. 1999;113:885–887. doi: 10.1017/s0022215100145505. [DOI] [PubMed] [Google Scholar]
- 50.Huang M, Ma D, Sun K, Yu G, Guo C, Gao F. Factors influencing survival rate in adenoid cystic carcinoma of the salivary glands. Int J Oral Maxillofac Surg. 1997;26:435–439. doi: 10.1016/s0901-5027(97)80008-2. [DOI] [PubMed] [Google Scholar]
- 51.Iannetti G, Valentini V, Rinna C, Ventucci E, Marianetti TM. Ethmoido-orbital tumors: our experience. J Craniofac Surg. 2005;16:1085–1091. doi: 10.1097/01.scs.0000164332.81428.ba. [DOI] [PubMed] [Google Scholar]
- 52.Issing PR, Hemmanouil I, Stover T, Kempf HG, Wilkens L, Heermann R, et al. Adenoid cystic carcinoma of the skull base. Skull Base Surg. 1999;9:271–275. doi: 10.1055/s-2008-1058137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jan GM, Wani MA, Goswami KC. Adenoid cystic carcinoma (ACC) of left sphenoidal sinus (case report) Indian J Pathol Microbiol. 1989;32:142–145. [PubMed] [Google Scholar]
- 54.Jansen EP, Keus RB, Hilgers FJ, Haas RL, Tan IB, Bartelink H. Does the combination of radiotherapy and debulking surgery favor survival in paranasal sinus carcinoma? Int J Radiat Oncol Biol Phys. 2000;48:27–35. doi: 10.1016/s0360-3016(00)00594-0. [DOI] [PubMed] [Google Scholar]
- 55.Katz TS, Mendenhall WM, Morris CG, Amdur RJ, Hinerman RW, Villaret DB. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck. 2002;24:821–829. doi: 10.1002/hed.10143. [DOI] [PubMed] [Google Scholar]
- 56.Kim GE, Park HC, Keum KC, Lee CG, Suh CO, Hur WJ, et al. Adenoid cystic carcinoma of the maxillary antrum. Am J Otolaryngol. 1999;20:77–84. doi: 10.1016/s0196-0709(99)90015-7. [DOI] [PubMed] [Google Scholar]
- 57.King DT, Cihak RW, Luther PK, Gurevitch AW, Hirose FM. Malignant neoplasms of the paranasal sinuses involving the skin. Arch Dermatol. 1978;114:1681–1683. [PubMed] [Google Scholar]
- 58.Kokemuller H, Eckardt A, Brachvogel P, Hausamen JE. Adenoid cystic carcinoma of the major and minor salivary glands. Retrospective analysis of 74 patients. Mund Kiefer Gesichtschir. 2003;7:94–101. doi: 10.1007/s10006-003-0461-4. [DOI] [PubMed] [Google Scholar]
- 59.Konno A, Ishikawa K, Numata T, Nagata H, Terada N, Okamoto Y. Analysis of factors affecting long-term treatment results of adenoid cystic carcinoma of the nose and paranasal sinuses. Acta Otolaryngol Suppl. 1998;537:67–74. doi: 10.1080/00016489850182387. [DOI] [PubMed] [Google Scholar]
- 60.Kowalski PJ, Paulino AF. Perineural invasion in adenoid cystic carcinoma: its causation/promotion by brain-derived neurotrophic factor. Hum Pathol. 2002;33:933–936. doi: 10.1053/hupa.2002.128249. [DOI] [PubMed] [Google Scholar]
- 61.Kraus DH, Roberts JK, Medendorp SV, Levine HL, Wood BG, Tucker HM, et al. Nonsquamous cell malignancies of the paranasal sinuses. Ann Otol Rhinol Laryngol. 1990;99:5–11. doi: 10.1177/000348949009900102. [DOI] [PubMed] [Google Scholar]
- 62.Kwon RO, Lyon DB, Floyd M, Girod DA. Sinonasal adenoid cystic carcinoma presenting as an orbital mass. Ophthal Plast Reconstr Surg. 2010;26:54–56. doi: 10.1097/IOP.0b013e3181b8ed49. [DOI] [PubMed] [Google Scholar]
- 63.Le QT, Birdwell S, Terris DJ, Gabalski EC, Varghese A, Fee W, Jr, et al. Postoperative irradiation of minor salivary gland malignancies of the head and neck. Radiother Oncol. 1999;52:165–171. doi: 10.1016/s0167-8140(99)00084-5. [DOI] [PubMed] [Google Scholar]
- 64.Lee DJ, Smith RR, Spaziani JT, Rostock R, Holliday M, Moses H. Adenoid cystic carcinoma of the nasopharynx. Case reports and literature review. Ann Otol Rhinol Laryngol. 1985;94:269–272. [PubMed] [Google Scholar]
- 65.Lee WJ, Kapadia SB, Stack BC., Jr Sinonasal adenoid cystic carcinoma with widespread symptomatic bony metastasis. Ear Nose Throat J. 2004;83:271–273. [PubMed] [Google Scholar]
- 66.Lloyd S, Yu JB, Wilson LD, Decker RH. Determinants and patterns of survival in adenoid cystic carcinoma of the head and neck, including an analysis of adjuvant radiation therapy. Am J Clin Oncol. 2011;34:76–81. doi: 10.1097/COC.0b013e3181d26d45. [DOI] [PubMed] [Google Scholar]
- 67.Loh KS, Barker E, Bruch G, O’Sullivan B, Brown DH, Goldstein DP, et al. Prognostic factors in malignancy of the minor salivary glands. Head Neck. 2009;31:58–63. doi: 10.1002/hed.20924. [DOI] [PubMed] [Google Scholar]
- 68.Lopez-Gines C, Cerda-Nicolas M, Llombart-Bosch A. Cytogenetic findings in a new case of adenoid cystic carcinoma arising in sphenoidal sinus. Cancer Genet Cytogenet. 1994;75:150–152. doi: 10.1016/0165-4608(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 69.Madison ML, Sorenson JM, Samant S, Robertson JH. The treatment of advanced sinonasal malignancies with pre-operative intra-arterial cisplatin and concurrent radiation. J Neurooncol. 2005;72:67–75. doi: 10.1007/s11060-004-2712-0. [DOI] [PubMed] [Google Scholar]
- 70.Mann W, Schuler-Voith C. Tumors of the paranasal sinuses and the nose: a retrospective study in 136 patients. Rhinology. 1983;21:173–177. [PubMed] [Google Scholar]
- 71.McMillan MD, Anderson G. Maxillary adenoid cystic carcinoma with annulate lamellae. Ultrastruct Pathol. 1994;18:493–498. doi: 10.3109/01913129409023224. [DOI] [PubMed] [Google Scholar]
- 72.Miller CL, Offutt WN, Kielar RA. Adenoid cystic carcinoma of the antrum and epiphora. Am J Ophthalmol. 1977;83:582–586. doi: 10.1016/0002-9394(77)90571-2. [DOI] [PubMed] [Google Scholar]
- 73.Miller RH, Calcaterra TC. Adenoid cystic carcinoma of the nose, paranasal sinuses, and palate. Arch Otolaryngol. 1980;106:424–426. doi: 10.1001/archotol.1980.00790310048012. [DOI] [PubMed] [Google Scholar]
- 74.Myers LL, Nussenbaum B, Bradford CR, Teknos TN, Esclamado RM, Wolf GT. Paranasal sinus malignancies: an 18-year single institution experience. Laryngoscope. 2002;112:1964–1969. doi: 10.1097/00005537-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Naficy S, Disher MJ, Esclamado RM. Adenoid cystic carcinoma of the paranasal sinuses. Am J Rhinol. 1999;13:311–314. doi: 10.2500/105065899782102872. [DOI] [PubMed] [Google Scholar]
- 76.Nagao T, Gaffey TA, Serizawa H, Sugano I, Ishida Y, Yamazaki K, et al. Dedifferentiated adenoid cystic carcinoma: a clinicopathologic study of 6 cases. Mod Pathol. 2003;16:1265–1272. doi: 10.1097/01.MP.0000097366.88165.08. [DOI] [PubMed] [Google Scholar]
- 77.Nordgard S, Franzen G, Boysen M, Halvorsen TB. Ki-67 as a prognostic marker in adenoid cystic carcinoma assessed with the monoclonal antibody MIB1 in paraffin sections. Laryngoscope. 1997;107:531–536. doi: 10.1097/00005537-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 78.Oppenheim H, Landau GH, Dorman DW, Mojsejenko I, Sleadd FB. Cylindroma involving the paranasal sinuses. Eye Ear Nose Throat Mon. 1968;47:669–678. [PubMed] [Google Scholar]
- 79.Ozsaran Z, Yalman D, Baltalarli B, Anacak Y, Esassolak M, Haydaroglu A. Radiotherapy in maxillary sinus carcinomas: evaluation of 79 cases. Rhinology. 2003;41:44–48. [PubMed] [Google Scholar]
- 80.Panchal L, Vaideeswar P, Kathpal D, Madiwale CV, Prabhat DP. Sino-nasal epithelial tumours: a pathological study of 69 cases. J Postgrad Med. 2005;51:30–35. [PubMed] [Google Scholar]
- 81.Parsons JT, Mendenhall WM, Mancuso AA, Cassisi NJ, Million RR. Malignant tumors of the nasal cavity and ethmoid and sphenoid sinuses. Int J Radiat Oncol Biol Phys. 1988;14:11–22. doi: 10.1016/0360-3016(88)90044-2. [DOI] [PubMed] [Google Scholar]
- 82.Perez AJ, Goyal P. Adenoid cystic carcinoma of the nasal cavity: a case report. Ear Nose Throat J. 2012;91:E22–E24. [PubMed] [Google Scholar]
- 83.Perusse R. Adenoid cystic carcinoma of the maxillary sinus. J Am Dent Assoc. 1997;128:1551–1555. doi: 10.14219/jada.archive.1997.0095. [DOI] [PubMed] [Google Scholar]
- 84.Pitman KT, Prokopakis EP, Aydogan B, Segas J, Carrau RL, Snyderman CH, et al. The role of skull base surgery for the treatment of adenoid cystic carcinoma of the sinonasal tract. Head Neck. 1999;21:402–407. doi: 10.1002/(sici)1097-0347(199908)21:5<402::aid-hed4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 85.Poetker DM, Toohill RJ, Loehrl TA, Smith TL. Endoscopic management of sinonasal tumors: a preliminary report. Am J Rhinol. 2005;19:307–315. [PubMed] [Google Scholar]
- 86.Pommier P, Liebsch NJ, Deschler DG, Lin DT, McIntyre JF, Barker FG, et al. Proton beam radiation therapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:1242–1249. doi: 10.1001/archotol.132.11.1242. [DOI] [PubMed] [Google Scholar]
- 87.Ramsden D, Sheridan BF, Newton NC, De Wilde FW. Adenoid cystic carcinoma of the head and neck: a report of 30 cases. Aust N Z J Surg. 1973;43:102–108. doi: 10.1111/j.1445-2197.1973.tb07319.x. [DOI] [PubMed] [Google Scholar]
- 88.Rudralingam M, Jones K, Woolford TJ. The unilateral opaque maxillary sinus on computed tomography. Br J Oral Maxillofac Surg. 2002;40:504–507. doi: 10.1016/s0266-4356(02)00225-5. [DOI] [PubMed] [Google Scholar]
- 89.Sadeghi A, Tran LM, Mark R, Sidrys J, Parker RG. Minor salivary gland tumors of the head and neck: treatment strategies and prognosis. Am J Clin Oncol. 1993;16:3–8. doi: 10.1097/00000421-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 90.Sato K, Ueda Y, Sakurai A, Ishikawa Y, Kaji S, Nojima T, et al. Adenoid cystic carcinoma of the maxillary sinus with gradual histologic transformation to high-grade adenocarcinoma: a comparative report with dedifferentiated carcinoma. Virchows Arch. 2006;448:204–208. doi: 10.1007/s00428-005-0054-8. [DOI] [PubMed] [Google Scholar]
- 91.Schramm VL, Jr, Imola MJ. Management of nasopharyngeal salivary gland malignancy. Laryngoscope. 2001;111:1533–1544. doi: 10.1097/00005537-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 92.Schulz-Ertner D, Didinger B, Nikoghosyan A, Jakel O, Zuna I, Wannenmacher M, et al. Optimization of radiation therapy for locally advanced adenoid cystic carcinomas with infiltration of the skull base using photon intensity-modulated radiation therapy (IMRT) and a carbon ion boost. Strahlenther Onkol. 2003;179:345–351. doi: 10.1007/s00066-003-1071-7. [DOI] [PubMed] [Google Scholar]
- 93.Shotton JC, Schmid S, Fisch U. The infratemporal fossa approach for adenoid cystic carcinoma of the skull base and nasopharynx. Otolaryngol Clin North Am. 1991;24:1445–1464. [PubMed] [Google Scholar]
- 94.Sivaji N, Basavaraj S, Stewart W, Dempster J. Adenoid cystic carcinoma of the nasal septum. Rhinology. 2003;41:253–254. [PubMed] [Google Scholar]
- 95.Sofferman RA, Heisse JW., Jr Adenoid cystic carcinoma of the nasopharynx after previous adenoid irradiation. Laryngoscope. 1985;95:458–461. [PubMed] [Google Scholar]
- 96.Solares CA, Fakhri S, Batra PS, Lee J, Lanza DC. Transnasal endoscopic resection of lesions of the clivus: a preliminary report. Laryngoscope. 2005;115:1917–1922. doi: 10.1097/01.mlg.0000172070.93173.92. [DOI] [PubMed] [Google Scholar]
- 97.Spiro RH, Koss LG, Hajdu SI, Strong EW. Tumors of minor salivary origin. A clinicopathologic study of 492 cases. Cancer. 1973;31:117–129. doi: 10.1002/1097-0142(197301)31:1<117::aid-cncr2820310116>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 98.Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma: factors influencing survival. Am J Surg. 1979;138:579–583. doi: 10.1016/0002-9610(79)90423-9. [DOI] [PubMed] [Google Scholar]
- 99.Strick MJ, Kelly C, Soames JV, McLean NR. Malignant tumours of the minor salivary glands–a 20 year review. Br J Plast Surg. 2004;57:624–631. doi: 10.1016/j.bjps.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 100.Sung MW, Kim KH, Kim JW, Min YG, Seong WJ, Roh JL, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2003;129:1193–1197. doi: 10.1001/archotol.129.11.1193. [DOI] [PubMed] [Google Scholar]
- 101.Takagi D, Fukuda S, Furuta Y, Yagi K, Homma A, Nagahashi T, et al. Clinical study of adenoid cystic carcinoma of the head and neck. Auris Nasus Larynx. 2001;28(Suppl):S99–102. doi: 10.1016/s0385-8146(01)00073-6. [DOI] [PubMed] [Google Scholar]
- 102.Takahashi T, Mitsuhashi N, Sakurai H, Takahashi M, Hirato J, Niibe H. A case report of adenoid cystic carcinoma of the nasopharynx with aggressive systemic bone metastases. Radiat Med. 1995;13:301–303. [PubMed] [Google Scholar]
- 103.Taylor JS, Cooley HN, Hicks JJ. Cylindroma of the maxillary antrum. Laryngoscope. 1965;75:1727–1736. doi: 10.1288/00005537-196511000-00005. [DOI] [PubMed] [Google Scholar]
- 104.Thorup C, Sebbesen L, Dano H, Leetmaa M, Andersen M, Buchwald C, et al. Carcinoma of the nasal cavity and paranasal sinuses in Denmark 1995–2004. Acta Oncol. 2010;49:389–394. doi: 10.3109/02841860903428176. [DOI] [PubMed] [Google Scholar]
- 105.Toluie S, Thompson LD. Sinonasal tract adenoid cystic carcinoma ex-pleomorphic adenoma: a clinicopathologic and immunophenotypic study of 9 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2012;6:409–421. doi: 10.1007/s12105-012-0381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tran L, Sidrys J, Horton D, Sadeghi A, Parker RG. Malignant salivary gland tumors of the paranasal sinuses and nasal cavity. The UCLA experience. Am J Clin Oncol. 1989;12:387–392. doi: 10.1097/00000421-198910000-00005. [DOI] [PubMed] [Google Scholar]
- 107.Tufano RP, Mokadam NA, Montone KT, Weinstein GS, Chalian AA, Wolf PF, et al. Malignant tumors of the nose and paranasal sinuses: hospital of the University of Pennsylvania experience 1990–1997. Am J Rhinol. 1999;13:117–123. doi: 10.2500/105065899782106698. [DOI] [PubMed] [Google Scholar]
- 108.Vedrine PO, Thariat J, Merrot O, Percodani J, Dufour X, Choussy O, et al. Primary cancer of the sphenoid sinus–a GETTEC study. Head Neck. 2009;31:388–397. doi: 10.1002/hed.20966. [DOI] [PubMed] [Google Scholar]
- 109.Veillon BJ, Raila FA, Fratkin JD, Russell WF. Magnetic resonance imaging of massive intracranial invasion by an ethmoidal adenoid cystic carcinoma (cylindroma) South Med J. 1996;89:321–323. doi: 10.1097/00007611-199603000-00012. [DOI] [PubMed] [Google Scholar]
- 110.Wakisaka S, Nonaka A, Morita Y, Fukui M, Kinoshita K. Adenoid cystic carcinoma with intracranial extension: report of three cases. Neurosurgery. 1990;26:1060–1065. doi: 10.1097/00006123-199006000-00025. [DOI] [PubMed] [Google Scholar]
- 111.Wang CC, See LC, Hong JH, Tang SG. Nasopharyngeal adenoid cystic carcinoma: five new cases and a literature review. J Otolaryngol. 1996;25:399–403. [PubMed] [Google Scholar]
- 112.Weber AL, Stanton AC. Malignant tumors of the paranasal sinuses: radiologic, clinical, and histopathologic evaluation of 200 cases. Head Neck Surg. 1984;6:761–776. doi: 10.1002/hed.2890060310. [DOI] [PubMed] [Google Scholar]
- 113.Wiseman SM, Popat SR, Rigual NR, Hicks WL, Jr, Orner JB, Wein RO, et al. Adenoid cystic carcinoma of the paranasal sinuses or nasal cavity: a 40-year review of 35 cases. Ear Nose Throat J. 2002;81:510–517. [PubMed] [Google Scholar]
- 114.Wolff AP, Ossoff RH, Clemis JD. Four unusual neoplasms of the nasopharynx. Otolaryngol Head Neck Surg. 1980;88:753–759. doi: 10.1177/019459988008800623. [DOI] [PubMed] [Google Scholar]
- 115.Wolfowitz BL, Schmaman A. Unusual malignant tumours of the maxillary sinuses. S Afr Med J. 1975;49:387–391. [PubMed] [Google Scholar]
- 116.Wolfowitz BL. Adenoid cystic carcinoma of the paranasal sinuses. S Afr Med J. 1971;45:972–974. [PubMed] [Google Scholar]
- 117.Wright ED, Raza SN, Heathcote JG. Atypical diagnostic, endoscopic, and pathologic findings in a case of adenoid cystic carcinoma arising from the sphenoid sinus. J Otolaryngol. 2005;34:226–230. doi: 10.2310/7070.2005.34402. [DOI] [PubMed] [Google Scholar]
- 118.Yin ZY, Wu XL, Hu YH, Gu XZ. Cylindroma of the nasopharynx: a chronic disease. Int J Radiat Oncol Biol Phys. 1986;12:25–30. doi: 10.1016/0360-3016(86)90411-6. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Q, Yang L, Yang AK, Guo ZM. Clinical study on 88 cases of adenoid cystic carcinoma in nasal cavity and paranasal sinuses. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;44:311–314. [PubMed] [Google Scholar]
- 120.Kuo YL, Tu TY, Chang CF, Li WY, Chang SY, Shiao AS, et al. Extra-major salivary gland pleomorphic adenoma of the head and neck: a 10-year experience and review of the literature. Eur Arch Otorhinolaryngol. 2011;268:1035–1040. doi: 10.1007/s00405-010-1437-2. [DOI] [PubMed] [Google Scholar]
- 121.Shukla NK, Hazarika S, Deo S, Kar M, Kumar S, Samaiya A, et al. Salivary gland tumours: profile and management at a tertiary cancer centre. J Indian Med Assoc. 2011;109:381–385. [PubMed] [Google Scholar]
- 122.Iyer NG, Kim L, Nixon IJ, Palmer F, Kraus D, Shaha AR, et al. Factors predicting outcome in malignant minor salivary gland tumors of the oropharynx. Arch Otolaryngol Head Neck Surg. 2010;136:1240–1247. doi: 10.1001/archoto.2010.213. [DOI] [PubMed] [Google Scholar]
- 123.Licitra L, Locati LD, Bossi P, Cantu G. Head and neck tumors other than squamous cell carcinoma. Curr Opin Oncol. 2004;16:236–241. doi: 10.1097/00001622-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 124.Lewis JE, Olsen KD, Sebo TJ. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol. 2001;32:596–604. doi: 10.1053/hupa.2001.25000. [DOI] [PubMed] [Google Scholar]
- 125.Nordkvist A, Roijer E, Bang G, Gustafsson H, Behrendt M, Ryd W, et al. Expression and mutation patterns of p53 in benign and malignant salivary gland tumors. Int J Oncol. 2000;16:477–483. doi: 10.3892/ijo.16.3.477. [DOI] [PubMed] [Google Scholar]
- 126.Gnepp DR. Malignant mixed tumors of the salivary glands: a review. Pathol Annu. 1993;28(Pt 1):279–328. [PubMed] [Google Scholar]
- 127.Batsakis JG. Neoplasms of the minor and lesser major salivary glands. Surg Gynecol Obstet. 1972;135:289–298. [PubMed] [Google Scholar]
- 128.Spiro RH, Huvos AG. Stage means more than grade in adenoid cystic carcinoma. Am J Surg. 1992;164:623–628. doi: 10.1016/s0002-9610(05)80721-4. [DOI] [PubMed] [Google Scholar]
- 129.Batsakis JG, Rice DH, Solomon AR. The pathology of head and neck tumors: squamous and mucous-gland carcinomas of the nasal cavity, paranasal sinuses, and larynx, part 6. Head Neck Surg. 1980;2:497–508. doi: 10.1002/hed.2890020610. [DOI] [PubMed] [Google Scholar]
- 130.Batsakis JG. Mucous gland tumors of the nose and paranasal sinuses. Ann Otol Rhinol Laryngol. 1970;79:557–562. doi: 10.1177/000348947007900319. [DOI] [PubMed] [Google Scholar]
- 131.Das S, Kirsch CF. Imaging of lumps and bumps in the nose: a review of sinonasal tumours. Cancer Imaging. 2005;5:167–177. doi: 10.1102/1470-7330.2005.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chang PC, Fischbein NJ, McCalmont TH, Kashani-Sabet M, Zettersten EM, Liu AY, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradiol. 2004;25:5–11. [PMC free article] [PubMed] [Google Scholar]
- 133.Lloyd G, Lund VJ, Howard D, Savy L. Optimum imaging for sinonasal malignancy. J Laryngol Otol. 2000;114:557–562. doi: 10.1258/0022215001906174. [DOI] [PubMed] [Google Scholar]
- 134.Farman AG, George DI, Jr, Clear RM. Computerized tomography of combined carcinomas arising in pleomorphic adenoma. Oral Surg Oral Med Oral Pathol. 1985;59:96–101. doi: 10.1016/0030-4220(85)90124-0. [DOI] [PubMed] [Google Scholar]
- 135.Calearo C, Pastore A, Merlo R. Cancer of the facial mass. Acta Otorhinolaryngol Ital. 1987;7(Suppl 14):1–27. [PubMed] [Google Scholar]
- 136.AJCC Cancer Staging Manual . 7th ed. New York: Springer; 2009. [Google Scholar]
- 137.Batsakis JG, Luna MA, El-Naggar A. Histopathologic grading of salivary gland neoplasms: III. Adenoid cystic carcinomas. Ann Otol Rhinol Laryngol. 1990;99:1007–1009. doi: 10.1177/000348949009901215. [DOI] [PubMed] [Google Scholar]
- 138.Szanto PA, Luna MA, Tortoledo ME, White RA. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984;54:1062–1069. doi: 10.1002/1097-0142(19840915)54:6<1062::aid-cncr2820540622>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 139.Moskaluk CA. Adenoid cystic carcinoma: clinical and molecular features. Head Neck Pathol. 2013;7:17–22. doi: 10.1007/s12105-013-0426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boland JM, McPhail ED, Garcia JJ, Lewis JE, Schembri-Wismayer DJ. Detection of human papilloma virus and p16 expression in high-grade adenoid cystic carcinoma of the head and neck. Mod Pathol. 2012;25:529–536. doi: 10.1038/modpathol.2011.186. [DOI] [PubMed] [Google Scholar]
- 141.Bishop JA, Ogawa T, Stelow EB, Moskaluk CA, Koch WM, Pai SI, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013;37:836–844. doi: 10.1097/PAS.0b013e31827b1cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ellis GL, Auclair PL. Tumors of the salivary glands. 3 Series. Washington: Armed Forces Institute of Pathology; 1995. [Google Scholar]
- 143.Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Hinerman RW, Villaret DB. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26:154–162. doi: 10.1002/hed.10380. [DOI] [PubMed] [Google Scholar]
- 144.Anderson TD, Feldman M, Weber RS, Ziober AF, Ziober BL. Tumor deposition of laminin-5 and the relationship with perineural invasion. Laryngoscope. 2001;111:2140–2143. doi: 10.1097/00005537-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 145.Katori H, Nozawa A, Tsukuda M. Increased expression of cyclooxygenase-2 and Ki-67 are associated with malignant transformation of pleomorphic adenoma. Auris Nasus Larynx. 2007;34:79–84. doi: 10.1016/j.anl.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 146.Chimona TS, Koutsopoulos AV, Malliotakis P, Nikolidakis A, Skoulakis C, Bizakis JG. Malignant mixed tumor of the nasal cavity. Auris Nasus Larynx. 2006;33:63–66. doi: 10.1016/j.anl.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 147.Wieneke JA, Thompson LD, Wenig BM. Basaloid squamous cell carcinoma of the sinonasal tract. Cancer. 1999;85:841–854. [PubMed] [Google Scholar]
- 148.Thompson LD. Olfactory neuroblastoma. Head Neck Pathol. 2009;3:252–259. doi: 10.1007/s12105-009-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barnes L, Ferlito A, Altavilla G, MacMillan C, Rinaldo A, Doglioni C. Basaloid squamous cell carcinoma of the head and neck: clinicopathological features and differential diagnosis. Ann Otol Rhinol Laryngol. 1996;105:75–82. doi: 10.1177/000348949610500115. [DOI] [PubMed] [Google Scholar]
- 150.Thompson LD, Seethala RR, Muller S. Ectopic Sphenoid Sinus Pituitary Adenoma (ESSPA) with Normal Anterior Pituitary Gland: a Clinicopathologic and Immunophenotypic Study of 32 Cases with a Comprehensive Review of the English Literature. Head Neck Pathol. 2012;6:75–100. doi: 10.1007/s12105-012-0336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Perzin KH, Gullane P, Clairmont AC. Adenoid cystic carcinomas arising in salivary glands: a correlation of histologic features and clinical course. Cancer. 1978;42:265–282. doi: 10.1002/1097-0142(197807)42:1<265::aid-cncr2820420141>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 152.Diaz EM, Kies MS. Chemotherapy for skull base cancers. Otolaryngol Clin North Am. 2001;34:1079–85. doi: 10.1016/s0030-6665(05)70366-2. [DOI] [PubMed] [Google Scholar]
- 153.Vrionis FD, Kienstra MA, Rivera M, Padhya TA. Malignant tumors of the anterior skull base. Cancer Control. 2004;11:144–151. doi: 10.1177/107327480401100302. [DOI] [PubMed] [Google Scholar]