Figure 1.
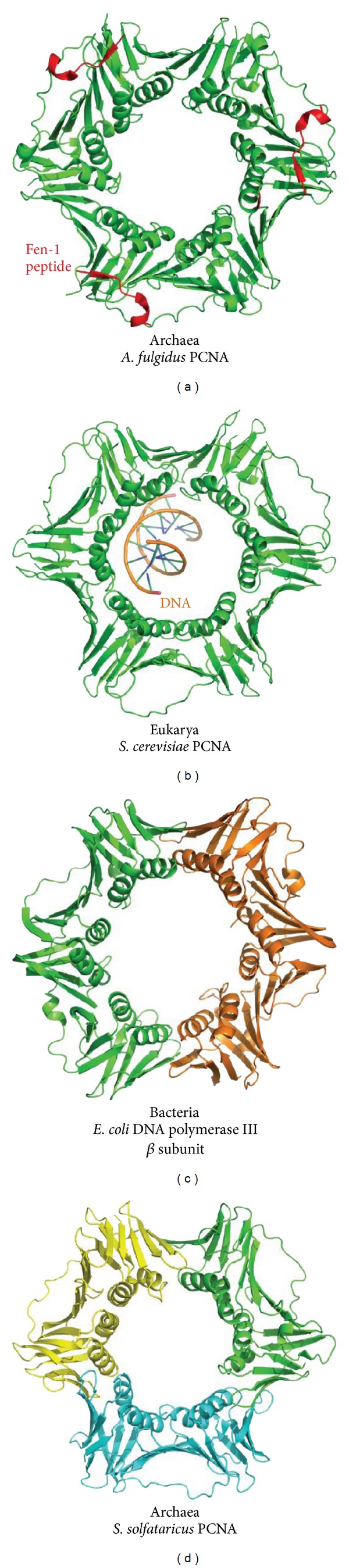
Comparison of PCNAs. The semblance of DNA sliding clamp proteins is made of differing general assemblies. (a) Archaeal A. fulgidus PCNA is a trimer composed of three identical subunits, which is the general case for PCNA proteins found in both archaea and eukaryotes. This particular structure has a FEN-1 peptide bound to each subunit (PDB code 1RXZ). PCNA proteins dock other enzymes to bring them into proximity to DNA when their functions are required. (b) The S. cerevisiae PCNA shares the general homotrimer assembly with other archaeal and eukaryotic PCNA proteins (PDB code 3K4X). This particular structure was engineered such that a DNA molecule was sequestered within the ring. (c) The β subunit of bacterial DNA polymerase III complexes shares the PCNA fold with its archaeal and eukaryotic counterparts. However, the assembly is formed by a homodimer (PDB code 2POL). (d) Archaeal S. solfataricus PCNA is unusual as it is assembled as a heterotrimer (PDB code 2HIK). It perhaps evolved this way to dock different enzymes with specificity.
