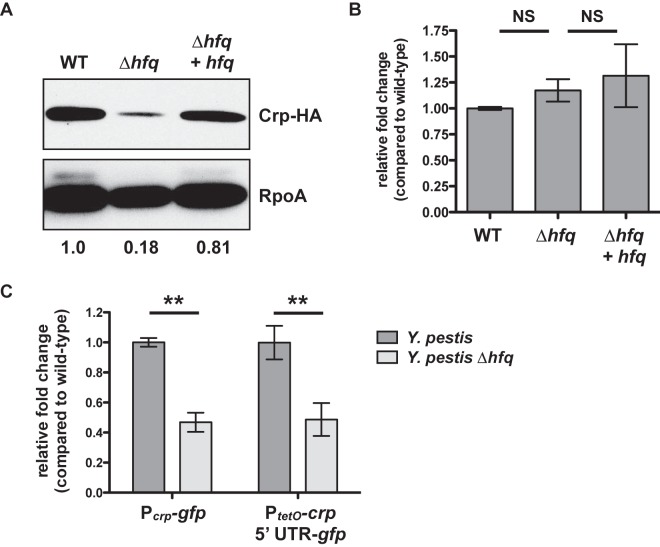
FIG 4 .
Hfq-dependent, posttranscriptional control of Crp. (A) Wild-type, ∆hfq, and Δhfq + hfq Y. pestis strains carrying an HA-tagged version of the crp gene were cultured for 6 h in BHI broth at 37°C, and whole-cell lysates were analyzed by immunoblotting with an anti-HA antibody. RpoA (bottom) is shown as a loading control. The relative density of the Crp-HA band compared with that of the wild type is shown below the RpoA panel. Data are representative of at least 3 independent experiments. (B) Steady-state levels of the crp transcript after 6 h at 37°C. Strains of Y. pestis were grown in triplicate, and the relative fold change of the crp transcript in the ∆hfq and Δhfq + hfq strains compared to that of the wild-type bacteria (set at 1) was determined by qRT-PCR using the ∆∆CT method. Data represent the combination of 3 independent experiments. (C) Wild-type or ∆hfq Y. pestis strains with the chromosomal-integrated Pcrp-gfp or PtetO-crp 5′ UTR-gfp reporter constructs were cultured at 37°C for 6 h, and fold change in fluorescence compared with that of the wild type (set at 1), normalized to the optical density of the culture, was determined. For the PtetO-crp 5′ UTR-gfp reporters, ATc was added at time 0. **, P < 0.005. Data represent the combination of 3 independent experiments. For all panels, error bars indicate standard deviation of the mean.