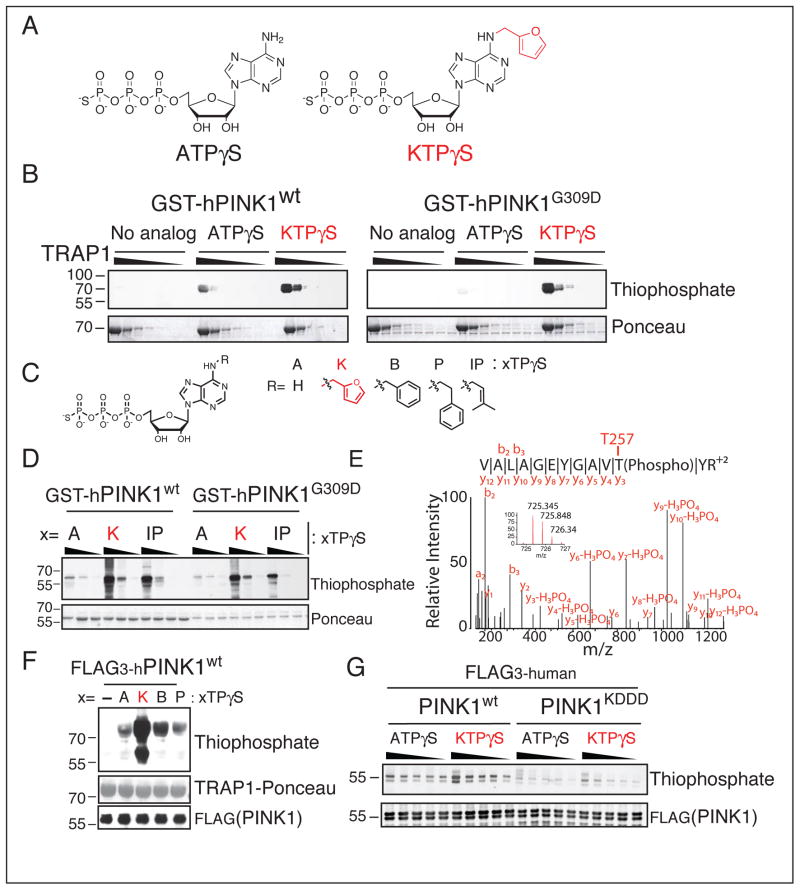
Figure 1. Neo-substrate Kinetin Triphosphate (KTP) amplifies PINK1 kinase activity in-vitro.
(A) Chemical structure of kinase substrate adenosine triphosphate with gamma thiophosphate (ATPγS) and neo-substrate kinetin triphosphate gamma thiophosphate (KTPγS).
(B–D) PINK1 kinase assay (B), with substrate 60–704TRAP1 (1 mg/ml decreasing by 1/3) and 500 μM indicated nucleotide (C) or (D), PINK1 alone (4.3 μM) 100, 200, 400 μM nucleotide analyzed by immunoblotting for thiophospho labeled TRAP1 and PINK1.
(E) PINK1 autophosphorylation site identified by specific peptide capture and LCMSMS found only with PINK1wt and KTPγS indicating this nucleotide is utilized as a bona fide substrate
(F) SF21 produced PINK1 was incubated with 60–704TRAP1 and the indicated nucleotide (structure shown in panel C) and analyzed as in (B–D)
(G) PINK1 kinase assay with indicated nucleotide (250 to 1250 μM in increments of 250 μM) analyzed as in (B–D) reveals much reduced phosphorylation activity with PINK1KDDD (lanes 11–20), increased autophosphorylation was seen with neo-substrate KTPγS over endogenous substrate ATPγS.