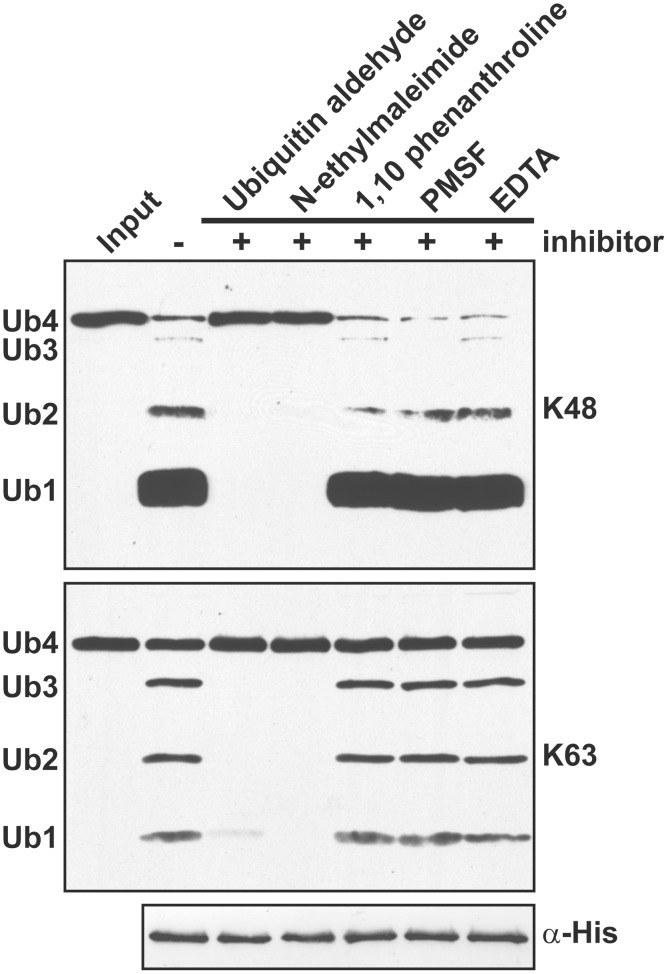
Figure 5.
A. thaliana OTU1 is catalytically inhibited by cysteine protease inhibitors. The effects of different protease inhibitors on the cleavage activity of His-tagged A. thaliana OTU1 (300 nM) for K48- and K63-linked UB tetramers (250 ng) were examined by comparing the resulting cleavage products after pre-incubation (5 min at 4°C) and the catalytic reaction (1 h at 37°C) in the absence (−) and presence (+) of 0.5 μM UB aldehyde, 0.5 μM N-ethylmaleimide, 0.5 μM 1,10-phenanthroline, 1 mM phenylmethylsulfonyl fluoride (PMSF), or 1 mM EDTA. The input substrate incubated without OTU1 was used as a negative control (Input). The input Ub4 and cleavage products Ub3, Ub2, and Ub1, labeled on the left, were detected by immunoblotting with α-UB. Duplicate samples were examined using antisera against the His-tag to confirm that OTU1 was input at equivalent levels (α-His).