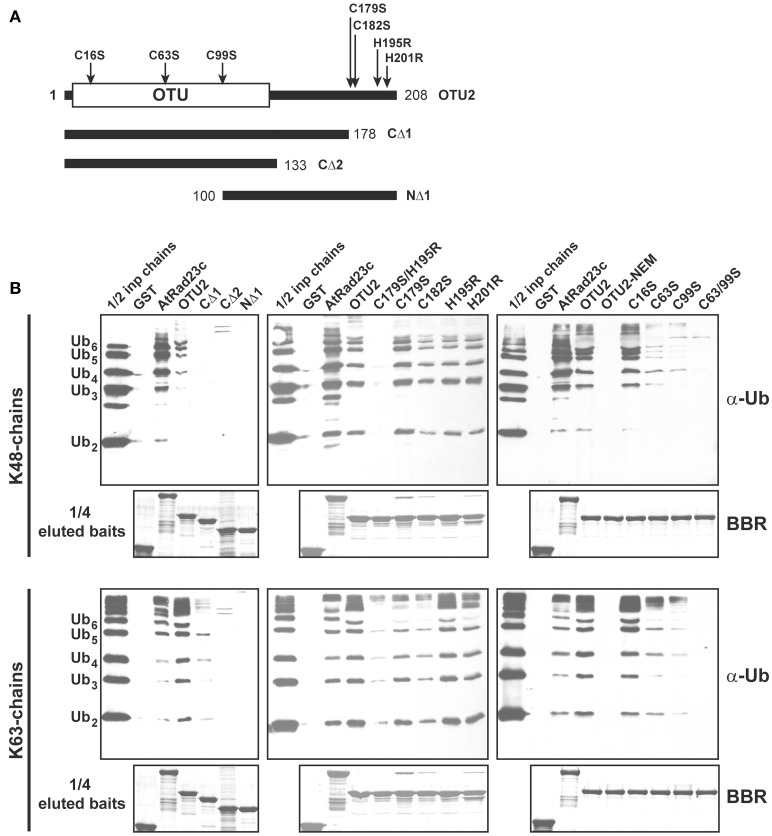
Figure 8.
Residues located at both the OTU2 N- and C-termini are critical for ubiquitin chain binding. (A) Schematic diagrams of the site-specific and deletion variants of OTU2 that were used to delineate the domains and residues involved in UB binding. The site-specific mutations and OTU domain are indicated. The numbers indicate the coordinates for the OTU2 variant termini. (B) GST pull-down products. Five micrograms of K48- or K63-linked UB chains (2–6 units, labeled on the left) were subjected to pull down by GST-fused OTU2 variants and negative and positive control proteins (GST and AtRAD23c, respectively). One microgram of the input chains (1/2 inp chains) and two-fifths of the eluted pulled-down products were analyzed by immunoblotting using antisera against human UB (α-Ub). One-tenth of the eluted pulled-down products (1/4 eluted baits) were visualized by Brilliant Blue R staining (BBR) to confirm equivalently immobilized baits. Wild-type OTU2 pre-incubated with 0.5 μM N-ethylmaleimide was analyzed to examine the involvement of catalytic and non-catalytic cysteine residues (OTU2-NEM).