Fig.4.
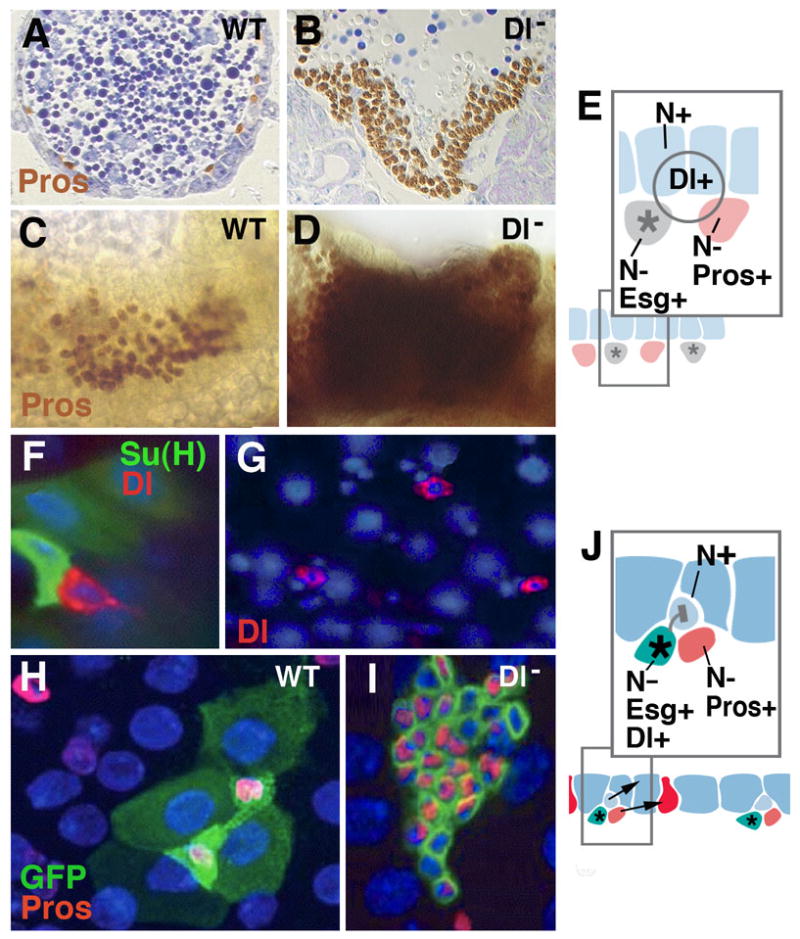
Notch signaling controls enteroendocrine cell formation at all stages of Drosophila development. A-E: Specification of larval endocrine and enterocyte precursors during embryogenesis. A, C: Section (A) and wholemount (C) of wild-type embryo, showing normal pattern of prospective endocrine cells (prospero-positive). B, D: Section (B) and wholemount (D) of Delta mutant embryo. Endocrine precursors are strongly increased at the expense of enterocytes. E: Model of Notch signaling function in embryonic midgut primordium. Fluctuations of the signaling Delta, widely expressed in the midgut primordium, result in cells that upregulate Notch and become epithelial larval enterocytes (blue); cells with low levels of Notch activity develop as prospective endocrine cells (red) and adult midgut progenitors (gray). The former upregulate the gene prospero (pros); the latter express the marker escargot (esg). F-J: adult midgut. Scattered stem cells express fluctuating levels of the signal Delta (red in F, G; from Ohlstein and Spradling, 2007). High levels of Delta activate Notch activity in neighboring, postmitotic enteroblast (monitored by Su(H), green in F). This will become enterocyte (shown in light blue in schematic of panel K). Lower levels of Delta in stem cell will fail to activate Notch in enteroblast, which thereby develops as endocrine cell (red in schematic J). H: Wild-type clone (labeled green), containing four enterocytes and two endocrine cells. Clones derived from stem cells lacking Delta (I) have increased number cells which all express endocrine fate (from Ohlstein and Spradling, 2007).
