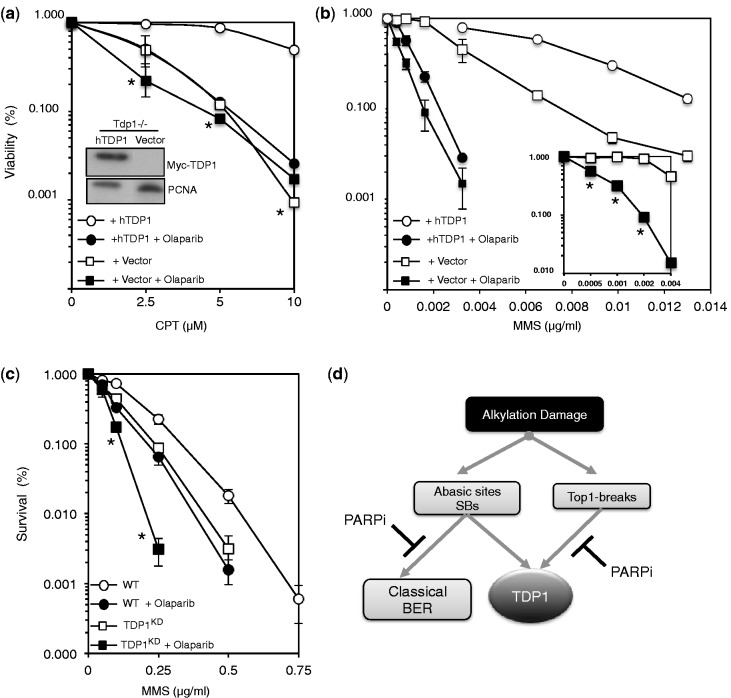
Figure 8.
The PARP inhibitor Olaparib sensitizes TDP1-deficient cells to alkylation damage but not Top1 poisons. (a) Chicken DT40 Tdp1−/− cells were stably transfected with an empty mammalian expression vector ‘Vector’ or with a construct expressing human Myc-TDP1 ‘hTDP1’. Cells were pre-incubated with 0.5 μM of the PARP inhibitor Olaparib for 1 h at 39°C followed by additional incubation of the indicated concentrations of CPT for 72 h at 39°C. Viability was determined by quantifying fluorescence signals using CellTiter-Blue. Viability of untreated cells was set to 100% and error bars represent standard error from three independent biological repeats. Inset: DT40 cell lysate fractionated by SDS–PAGE and analyzed by anti-Myc (9B11; Cell signaling) and anti-PCNA (PC10) immunoblotting. Asterisks denote statistical differences (P > 0.1; t-test) between Vector alone ‘open squares’ and Vector + Olaparib ‘closed squares’ (b) Viability of the indicated DT40 cells was analyzed in presence or absence of Olaparib as described earlier in text, following incubation with the indicated concentrations of MMS for 72 h. Data are the average of three independent repeats ± s.e.m. Asterisks denote statistical differences (P < 0.02; t-test) between Vector alone ‘open squares’ and Vector + Olaparib ‘closed squares’ Inset: survival curves as described earlier in the text following incubation with a lower dose range of MMS (c) WT and TDP1KD human MRC5 cells were pre-incubated with 1 μM Olaparib for 60 min followed by additional incubation with the indicated concentrations of MMS for 15 min at 37°C. Survival was determined from three independent repeats and data are the average ± s.e.m. Asterisks denote statistical differences (P < 0.01, t-test) between TDP1KD ‘open squares’ and TDP1KD + Olaparib ‘closed squares’ (d) Model for the repair of alkylation-induced DNA damage by TDP1. Base damage induces both Top1- and AP/3’-dRP DNA breaks. The former are dealt with TDP1 in a PARP-dependent process, whereas the latter are processed in a PARP-independent mechanism. PARP activity appears to promote TDP1 alternative pathways in response to alkylation damage, which likely involves canonical base excision repair factors such as APE1.