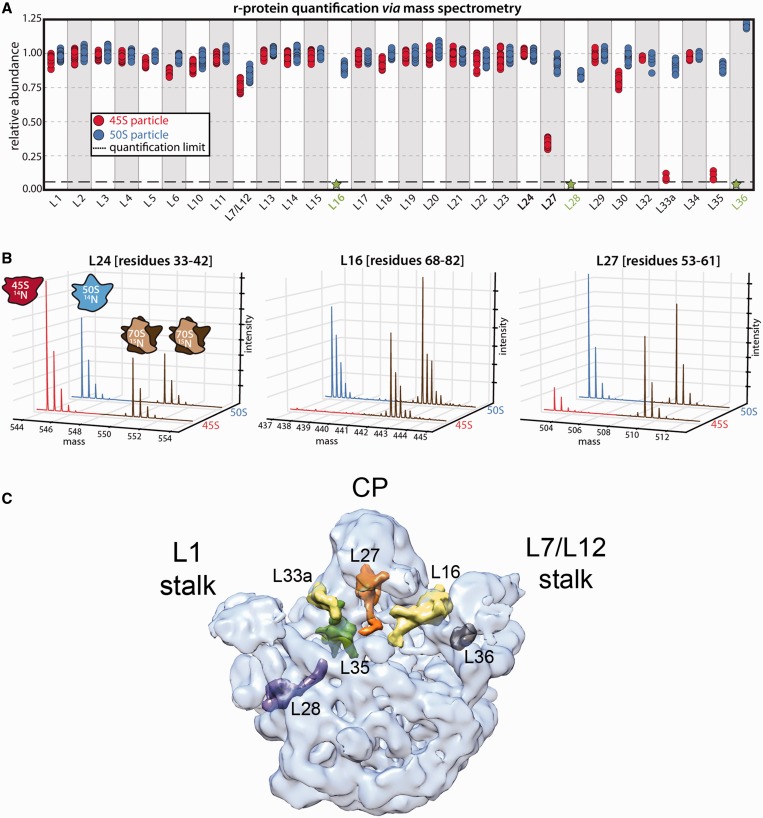
Figure 1.
Protein complement of the 45S particle. (A) Relative protein abundance in 14N-labeled 50S subunits (blue) and 45S particles (red) with respect to 15N-labeled 50S subunits from functional 70S ribosomes. The 50S subunits and 45S particles were purified from IF2-depleted and RbgA-depleted cells, respectively. The 70S subunits used as reference were purified from wild-type cells. Each marker represents a unique measurement of a peptide resulting from a tryptic digest of the parent protein. Each sample was analyzed in quadruplicate, and datasets were merged to improve proteomic coverage. (B) Representative mass chromatograms of proteins present stochiometrically (left), completely absent (middle) or depleted (right) from the 45S particle are shown in red, with mass chromatograms from the reference particles in brown. Equivalent chromatograms for the 50S subunits are shown in blue. (C) Transparent surface representation of the control cryo-EM map obtained for the mature 50S subunit. The X-ray structure from T. thermophilus (PDB ID 2Y11) was docked into the cryo-EM to show the r-proteins that were found absent or depleted in the 45S particles. Individual proteins are shown as a surface. The central protuberance is labeled as CP.