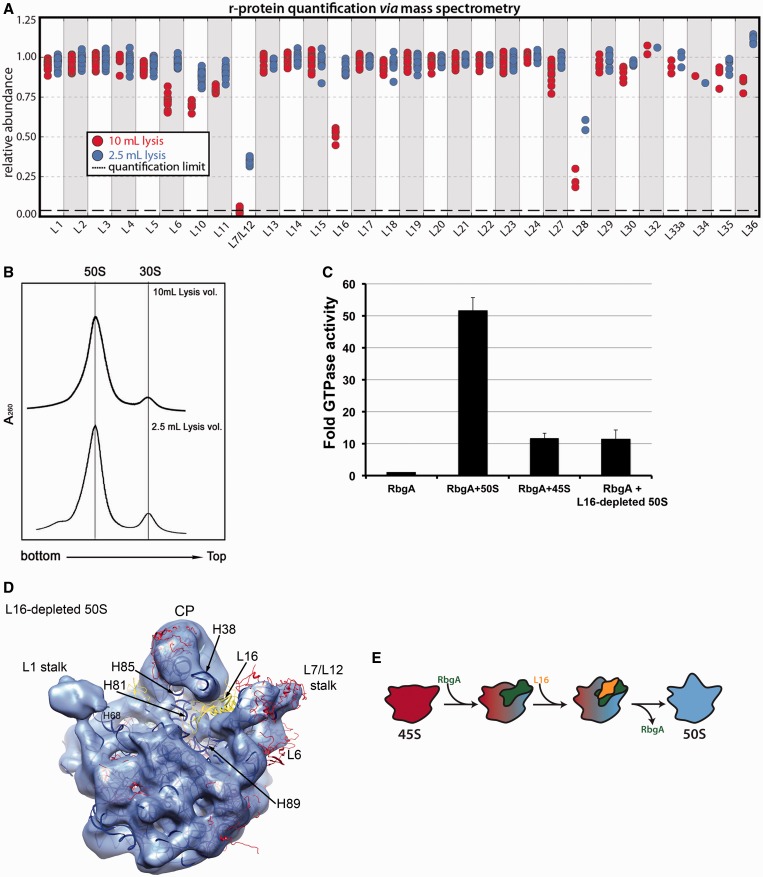
Figure 7.
Depletion of L16 from the mature 50S subunit partially reverts the subunit to an immature state. (A) Relative protein abundance in 14N-labeled 50S subunit purified using standard (2.5 ml; blue) or higher (10 ml; red) lysis volumes with respect to 15N-labeled 50S subunits from functional 70S ribosomes. The 50S subunits were purified from IF2-depleted cells (see methods). The 70S subunits used as reference were purified from wild-type cells. Each marker represents a unique measurement of a peptide resulting from a tryptic digest of the parent r-protein. Each sample was analyzed in duplicate and datasets were merged to improve proteomic coverage. 50S subunits purified using a higher lysis volume showed a specific depletion of L16, and these particles were called ‘L16-depleted 50S subunits’. (B) Sedimentation profiles of L16-depleted 50S subunits in sucrose gradients. Cells grown as described in (B) were lysed either in 10 ml buffer (top panel) or in 2.5 ml of buffer (bottom panel). Analysis of sedimentation profiles in a sucrose gradient revealed that in each instance, large subunit particles co-migrated at 50S. (C) GTPase activity of RbgA in the presence of L16-depleted 50S subunit, 50S subunits purified using standard lysis volume and 45S particles. In each case, the GTPase activity was normalized to that of the RbgA protein in isolation. Plotted values represent the average and standard deviation obtained from three replicas of each reaction. (D) Cryo-EM structure of the L16-depleted 50S subunit refined to 13 Å resolution (Supplementary Figure S12). The X-ray structure of the 50S subunit from T. thermophilus (PDB ID 2Y11) was docked in the cryo-EM map. L1 and L25/Ctc have been removed for clarity. L16, L6 and the rRNA helices that exhibited conformational deviations from the mature 50S subunit are labeled. The central protuberance is labeled as CP. (E) Model for the functional interplay between RgbA and L16 during the last stages of maturation of the 50S subunit. Under this model, RbgA directly or indirectly mediates binding of L16 to the 45S particle (red), which in turn releases RbgA (upon GTP hydrolysis). Binding of L16 and release of RbgA induce conformational changes that transition the assembling particle toward the mature structure (blue).