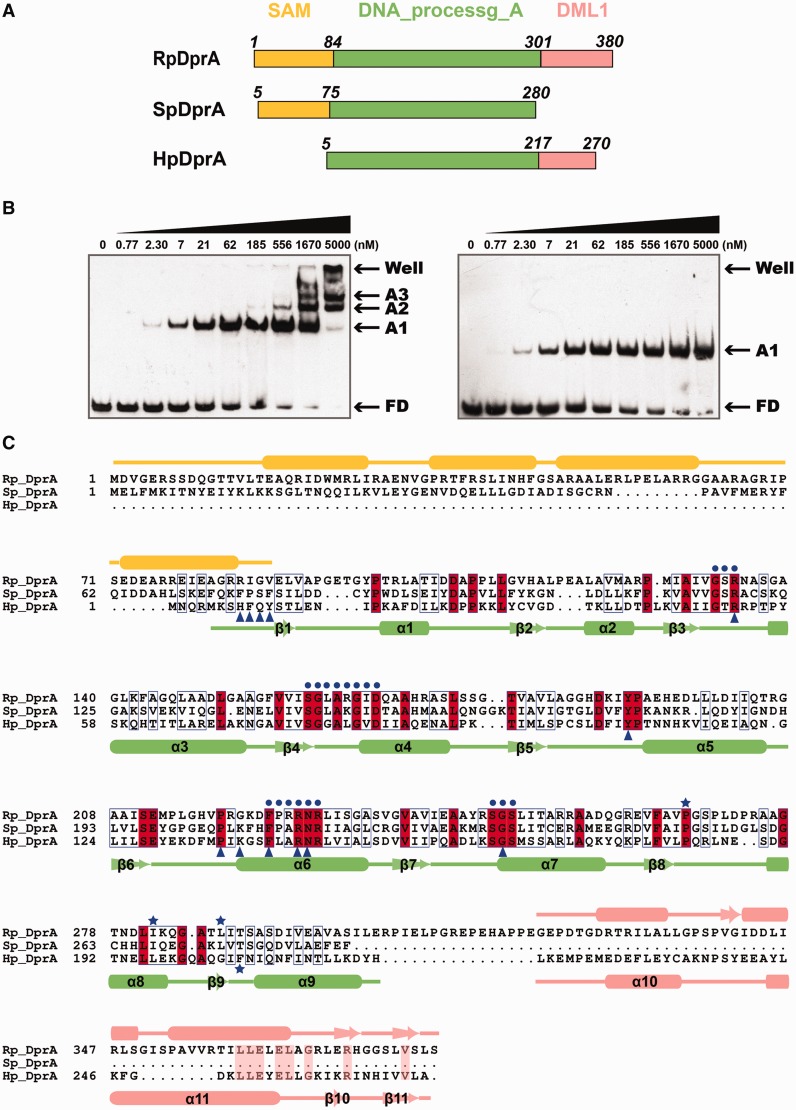
Figure 1.
Sequence alignment of three DprA homologous proteins from R. palustris, S. pneumonia and H. pylori. (A) Linear representation of the three DprA proteins with multi-domains, including SAM domain (orange), DNA_processg_A domain (pale green) and DML1 domain (salmon). (B) EMSA results of full-length HpDprA–dT35 (left panel) and HpDprA(5-225)–dT35 (right panel). Different concentrations of HpDprA (0.77 nM to 5 μM) with 5 nM biotin-labeled ssDNA are incubated for 20 min at 25°C before loading on the gel. Samples were electrophoresed on 8% PAGE and detected by fluorography. (C) Sequence alignment of three DprAs (color code as in (A)). Important residues are indicated by symbols in blue: residues involved in hydrophobic interaction of dimerization interface (stars), conserved residues near the binding pocket (dots) and residues directly contacted by ssDNA (triangles).