Abstract
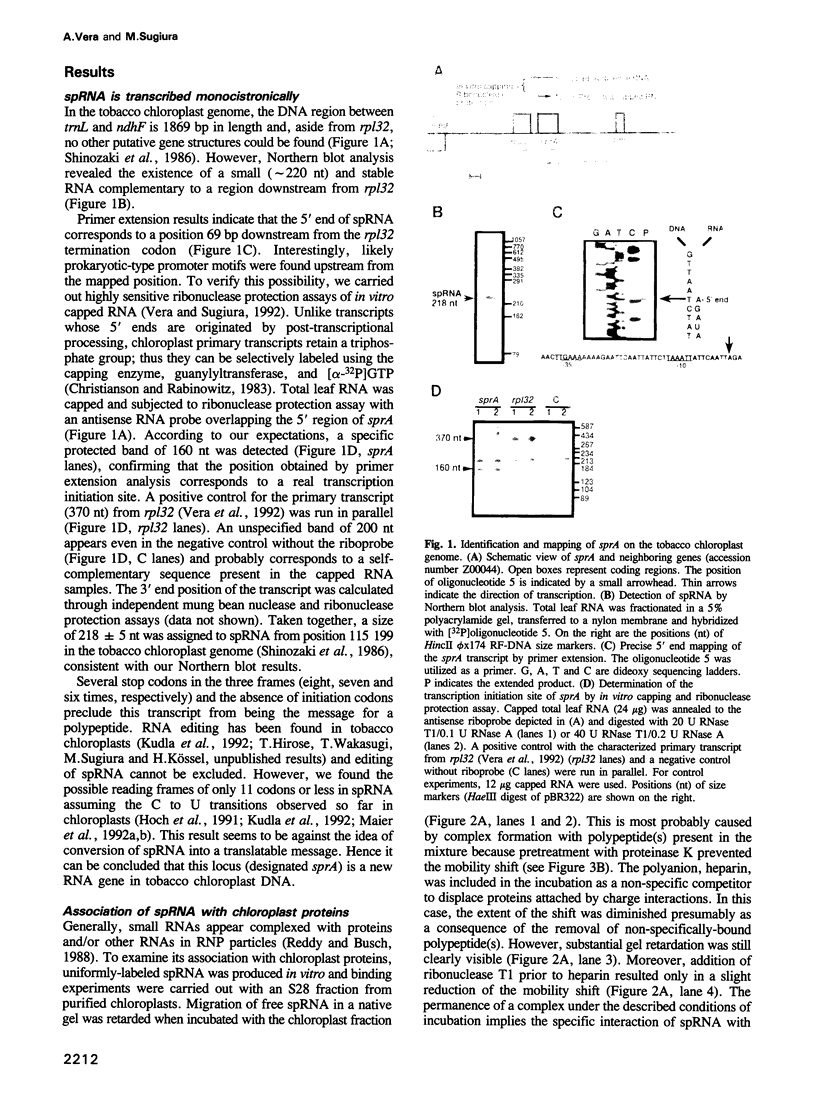
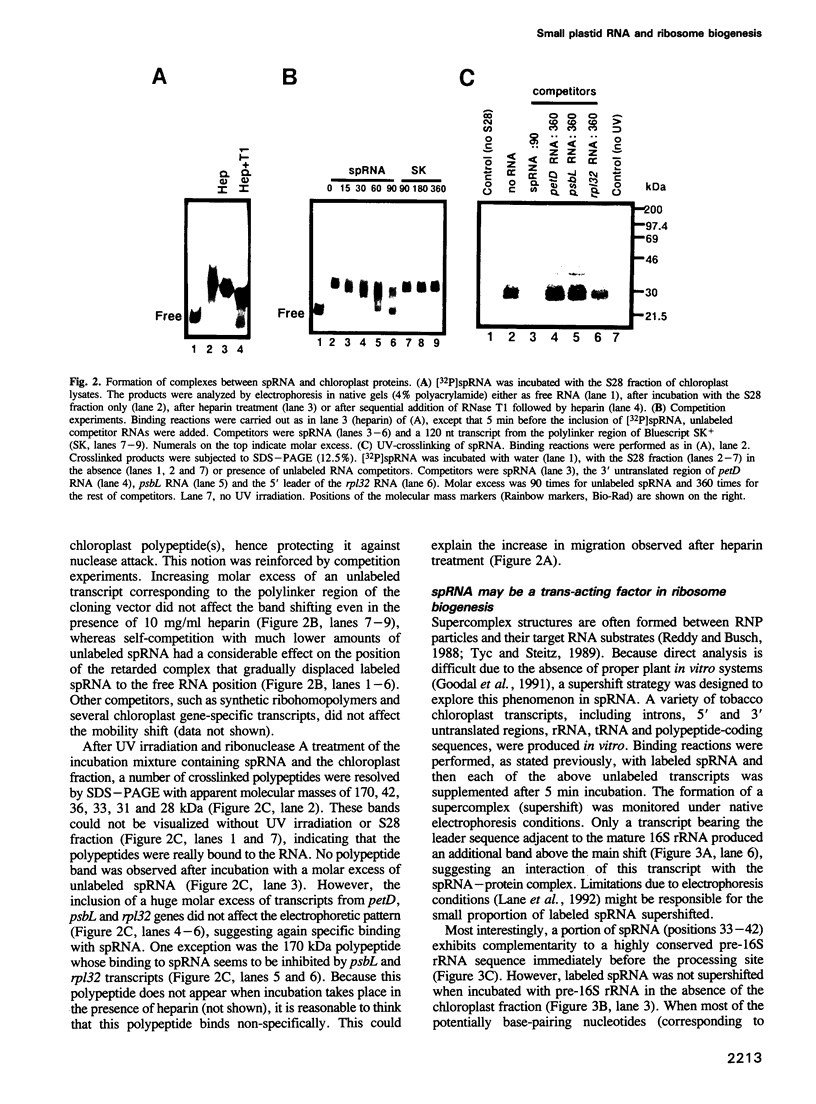
A small plastid-encoded RNA (spRNA, 218 nt) has been detected in tobacco. The corresponding locus (sprA) does not contain any open reading frame and is actively transcribed from its own promoter, as shown by ribonuclease protection assays using in vitro capped RNAs. Gel-shift and UV-crosslinking experiments showed the formation of a specific complex between spRNA and chloroplast polypeptides. The mobility of the complex was further shifted when a transcript bearing part of the 16S rRNA leader sequence was added to the incubation mixture. Glycerol gradient fractionation of a chloroplast lysate indicated a preferential sedimentation of spRNA at 15-20S and 70S. These observations, and the potential base-pairing with the leader sequence of pre-16S rRNA, suggest a role for spRNA in chloroplast ribosome biogenesis, i.e. 16S rRNA maturation. By sequencing of tomato plastid DNA and heterologous northern hybridizations, the presence of sprA homologs and their expression in a number of dicot plants have also been shown.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araya A., Domec C., Begu D., Litvak S. An in vitro system for the editing of ATP synthase subunit 9 mRNA using wheat mitochondrial extracts. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1040–1044. doi: 10.1073/pnas.89.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A. Nuclear Mutants of Maize with Defects in Chloroplast Polysome Assembly Have Altered Chloroplast RNA Metabolism. Plant Cell. 1993 Apr;5(4):389–402. doi: 10.1105/tpc.5.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrame M., Tollervey D. Identification and functional analysis of two U3 binding sites on yeast pre-ribosomal RNA. EMBO J. 1992 Apr;11(4):1531–1542. doi: 10.1002/j.1460-2075.1992.tb05198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T., Rabinowitz M. Identification of multiple transcriptional initiation sites on the yeast mitochondrial genome by in vitro capping with guanylyltransferase. J Biol Chem. 1983 Nov 25;258(22):14025–14033. [PubMed] [Google Scholar]
- Danon A., Mayfield S. P. Light regulated translational activators: identification of chloroplast gene specific mRNA binding proteins. EMBO J. 1991 Dec;10(13):3993–4001. doi: 10.1002/j.1460-2075.1991.tb04974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Swanson M. S., Piñol-Roma S. Heterogeneous nuclear ribonucleoprotein particles and the pathway of mRNA formation. Trends Biochem Sci. 1988 Mar;13(3):86–91. doi: 10.1016/0968-0004(88)90046-1. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Choquet Y., Girard-Bascou J., Michel F., Schirmer-Rahire M., Rochaix J. D. A small chloroplast RNA may be required for trans-splicing in Chlamydomonas reinhardtii. Cell. 1991 Apr 5;65(1):135–143. doi: 10.1016/0092-8674(91)90415-u. [DOI] [PubMed] [Google Scholar]
- Gruissem W. Chloroplast gene expression: how plants turn their plastids on. Cell. 1989 Jan 27;56(2):161–170. doi: 10.1016/0092-8674(89)90889-1. [DOI] [PubMed] [Google Scholar]
- Guthrie C. Messenger RNA splicing in yeast: clues to why the spliceosome is a ribonucleoprotein. Science. 1991 Jul 12;253(5016):157–163. doi: 10.1126/science.1853200. [DOI] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Hoch B., Maier R. M., Appel K., Igloi G. L., Kössel H. Editing of a chloroplast mRNA by creation of an initiation codon. Nature. 1991 Sep 12;353(6340):178–180. doi: 10.1038/353178a0. [DOI] [PubMed] [Google Scholar]
- Inouye M., Delihas N. Small RNAs in the prokaryotes: a growing list of diverse roles. Cell. 1988 Apr 8;53(1):5–7. doi: 10.1016/0092-8674(88)90480-1. [DOI] [PubMed] [Google Scholar]
- Kass S., Tyc K., Steitz J. A., Sollner-Webb B. The U3 small nucleolar ribonucleoprotein functions in the first step of preribosomal RNA processing. Cell. 1990 Mar 23;60(6):897–908. doi: 10.1016/0092-8674(90)90338-f. [DOI] [PubMed] [Google Scholar]
- Kudla J., Igloi G. L., Metzlaff M., Hagemann R., Kössel H. RNA editing in tobacco chloroplasts leads to the formation of a translatable psbL mRNA by a C to U substitution within the initiation codon. EMBO J. 1992 Mar;11(3):1099–1103. doi: 10.1002/j.1460-2075.1992.tb05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D., Prentki P., Chandler M. Use of gel retardation to analyze protein-nucleic acid interactions. Microbiol Rev. 1992 Dec;56(4):509–528. doi: 10.1128/mr.56.4.509-528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. D., Zagorski J., Fournier M. J. Depletion of U14 small nuclear RNA (snR128) disrupts production of 18S rRNA in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Mar;10(3):1145–1152. doi: 10.1128/mcb.10.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Q., Sugiura M. Three distinct ribonucleoproteins from tobacco chloroplasts: each contains a unique amino terminal acidic domain and two ribonucleoprotein consensus motifs. EMBO J. 1990 Oct;9(10):3059–3066. doi: 10.1002/j.1460-2075.1990.tb07502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. M., Hoch B., Zeltz P., Kössel H. Internal editing of the maize chloroplast ndhA transcript restores codons for conserved amino acids. Plant Cell. 1992 May;4(5):609–616. doi: 10.1105/tpc.4.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. M., Neckermann K., Hoch B., Akhmedov N. B., Kössel H. Identification of editing positions in the ndhB transcript from maize chloroplasts reveals sequence similarities between editing sites of chloroplasts and plant mitochondria. Nucleic Acids Res. 1992 Dec 11;20(23):6189–6194. doi: 10.1093/nar/20.23.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W. A binding consensus: RNA-protein interactions in splicing, snRNPs, and sex. Cell. 1989 Apr 7;57(1):1–3. doi: 10.1016/0092-8674(89)90164-5. [DOI] [PubMed] [Google Scholar]
- Maxwell E. S., Martin T. E. A low-molecular-weight RNA from mouse ascites cells that hybridizes to both 18S rRNA and mRNA sequences. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7261–7265. doi: 10.1073/pnas.83.19.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieszczak M., Klahre U., Levy J. H., Goodall G. J., Filipowicz W. Multiple plant RNA binding proteins identified by PCR: expression of cDNAs encoding RNA binding proteins targeted to chloroplasts in Nicotiana plumbaginifolia. Mol Gen Genet. 1992 Sep;234(3):390–400. doi: 10.1007/BF00538698. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D. Post-transcriptional steps in the expression of chloroplast genes. Annu Rev Cell Biol. 1992;8:1–28. doi: 10.1146/annurev.cb.08.110192.000245. [DOI] [PubMed] [Google Scholar]
- Schuster G., Gruissem W. Chloroplast mRNA 3' end processing requires a nuclear-encoded RNA-binding protein. EMBO J. 1991 Jun;10(6):1493–1502. doi: 10.1002/j.1460-2075.1991.tb07669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. RNA editing--a novel genetic phenomenon? Science. 1990 Oct 26;250(4980):512–513. doi: 10.1126/science.1700474. [DOI] [PubMed] [Google Scholar]
- Srivastava A. K., Schlessinger D. Processing pathway of Escherichia coli 16S precursor rRNA. Nucleic Acids Res. 1989 Feb 25;17(4):1649–1663. doi: 10.1093/nar/17.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M. The chloroplast chromosomes in land plants. Annu Rev Cell Biol. 1989;5:51–70. doi: 10.1146/annurev.cb.05.110189.000411. [DOI] [PubMed] [Google Scholar]
- Sugiura M. The chloroplast genome. Plant Mol Biol. 1992 May;19(1):149–168. doi: 10.1007/BF00015612. [DOI] [PubMed] [Google Scholar]
- Tyc K., Steitz J. A. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 1989 Oct;8(10):3113–3119. doi: 10.1002/j.1460-2075.1989.tb08463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera A., Matsubayashi T., Sugiura M. Active transcription from a promoter positioned within the coding region of a divergently oriented gene: the tobacco chloroplast rpl32 gene. Mol Gen Genet. 1992 May;233(1-2):151–156. doi: 10.1007/BF00587573. [DOI] [PubMed] [Google Scholar]
- Vera A., Sugiura M. Combination of in vitro capping and ribonuclease protection improves the detection of transcription start sites in chloroplasts. Plant Mol Biol. 1992 May;19(2):309–311. doi: 10.1007/BF00027352. [DOI] [PubMed] [Google Scholar]
- Vera A., Yokoi F., Sugiura M. The existence of pre-mature 16S rRNA species in plastid ribosomes. FEBS Lett. 1993 Jul 19;327(1):29–31. doi: 10.1016/0014-5793(93)81032-u. [DOI] [PubMed] [Google Scholar]
- Wolfe K. H., Katz-Downie D. S., Morden C. W., Palmer J. D. Evolution of the plastid ribosomal RNA operon in a nongreen parasitic plant: accelerated sequence evolution, altered promoter structure, and tRNA pseudogenes. Plant Mol Biol. 1992 Apr;18(6):1037–1048. doi: 10.1007/BF00047707. [DOI] [PubMed] [Google Scholar]
- Ye L. H., Li Y. Q., Fukami-Kobayashi K., Go M., Konishi T., Watanabe A., Sugiura M. Diversity of a ribonucleoprotein family in tobacco chloroplasts: two new chloroplast ribonucleoproteins and a phylogenetic tree of ten chloroplast RNA-binding domains. Nucleic Acids Res. 1991 Dec 11;19(23):6485–6490. doi: 10.1093/nar/19.23.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]