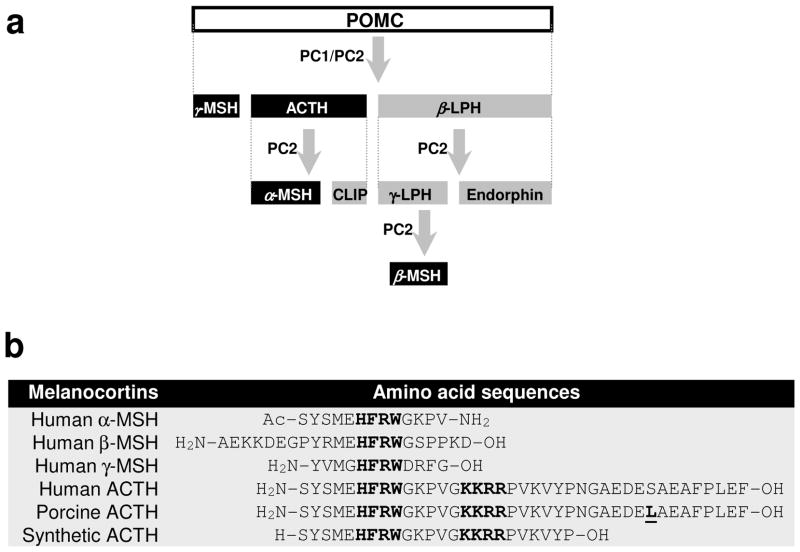
Figure 1. Biosynthesis and molecular structures of melanocortin peptides.
(a) Pituitary biosynthesis of melanocortins. Melanocortins are synthesized in the anterior pituitary gland from the precursor peptide pre-pro-opiomelanocortin (pre-POMC), which undergoes a series of post-translational modifications such as phosphorylation and glycosylation before it is proteolytically cleaved by prohormone convertase (PC) enzymes PC1 and PC2 to yield a chemically and biogenetically related family of melanocortin polypeptides, including ACTH, α-melanocyte stimulating hormone (MSH), β-MSH and γ-MSH. CLIP, corticotropin-like intermediate peptide; LPH, lipotropin; (b) Molecular sequences of natural human melanocortin peptides, porcine ACTH and synthetic ACTH. ACTH is a linear nonatriacontapeptide straight-chain polypeptide containing 39 amino acids. The sequence of amino acid occupying positions 25 to 33 may vary among species. However, the N-terminal 24-amino acid segment is highly conserved and actually identical in all species. The only one different amino acid between porcine and human ACTH is underscored (residual 31 is serine for human and lysine for porcine). All melanocortin peptides share the same core tetrapeptide sequence His-Phe-Arg-Trp (HFRW), which is the minimal sequence required for selectivity and stimulation of the cognate MCRs (except MC2R). The Lys-Lys-Arg-Arg (KKRR) motif is the address sequence that permits MC2R recognition and is unique to ACTH.