Figure 1.
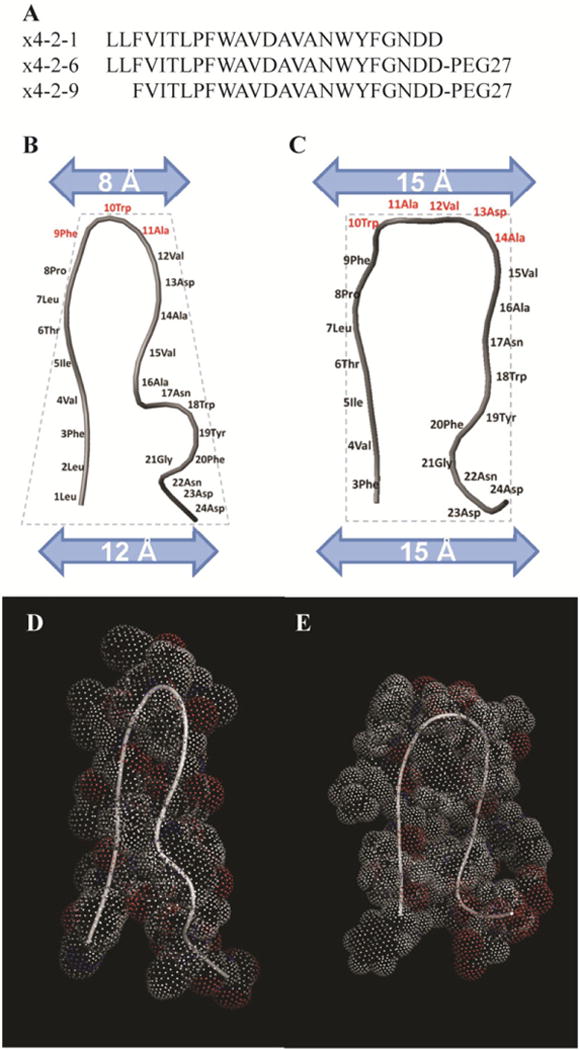
Structural characterization of x4-2-1, x4-2-6, and x4-2-9 peptides. (A) Amino acid sequences of x4-2-6 and x4-2-9 peptides differ by only two N-terminal residues. Comparison of average structures of x4-2-1 (B) and x4-2-9 (C) shows differences in the overall topology of peptide monomers. The width of the head and the base of the hairpin structures were measured. Residues involved in the head region are shown in dark gray. The dotted outline shows the conical shape of the x4-2-1 structure and the cylindrical shape of the x4-2-9 structure. The blue (light gray) bars represent the widths of the head and the base of x4-2-1 and x4-2-9 hairpins. The space filling models of average x4-2-1 and x4-2-9 structures are shown in (D) and (E), respectively.
