Abstract
BACKGROUND:
First-generation cephalosporins and antistaphylococcal penicillins are typically the first choice for treating skin and soft tissue infections (SSTI), but are not effective for infections caused by methicillin-resistant Staphylococcus aureus (MRSA). It is currently unclear what percentage of SSTIs is caused by community-associated MRSA in different regions in Canada.
OBJECTIVES:
To determine the incidence of MRSA in children presenting to a pediatric emergency department with SSTI, and to determine which antibiotics were used to treat these infections.
METHODS:
All visits to a pediatric emergency department were reviewed from April 15, 2010 to April 14, 2011. Diagnoses of cellulitis, abscess, impetigo, folliculitis and skin infection (not otherwise specified) were reviewed in detail to determine whether a culture was taken and which antibiotic was prescribed.
RESULTS:
There were 367 cases of SSTI diagnosed over the study period. Forty-five (12.3%) patients had lesions that were swabbed for culture and sensitivity. S aureus was the most common organism found, with 14 (66%) methicillin-sensitive cases and seven (33%) methicillin-resistant cases. Of the seven cases of MRSA identified, only one patient had clear risk factors for hospital-acquired MRSA. First-generation cephalosporins were initially prescribed for 280 (76%) patients.
CONCLUSIONS:
The overall incidence of MRSA in the population presenting to a pediatric emergency department in Newfoundland and Labrador appeared to be low, although only a small percentage of infections were cultured. At this time, there appears to be no need to change empirical antibiotic coverage, which remains a first-generation cephalosporin.
Keywords: Emergency medicine, Methicillin-resistant Staphylococcus aureus, Pediatrics, Skin and soft tissue infections
Abstract
HISTORIQUE :
Les céphalosporines de première génération et les pénicillines antistaphylococciques sont généralement le traitement de première intention des infections des tissus cutanés et des tissus mous (ITCM), mais ne sont pas efficaces contre les infections causées par le Staphylococcus aureus résistant à la méthicilline (SARM). On ne sait pas quel pourcentage d’ITCM est causé par un SARM d’origine non nosocomiale dans diverses régions du pays.
OBJECTIFS :
Déterminer l’incidence de SARM chez les enfants qui consultent à une salle d’urgence pédiatrique en raison d’une ITCM, ainsi que les antibiotiques utilisés pour traiter ces infections.
MÉTHODOLOGIE :
Les chercheurs ont analysé toutes les visites à la salle d’urgence effectuées entre le 15 avril 2010 et le 14 avril 2011. Ils ont examiné attentivement les diagnostics de cellulite, d’abcès, d’impétigo, de folliculite et d’infection cutanée (non autrement spécifiée) pour déterminer si une culture avait été effectuée et quel antibiotique avait été prescrit.
RÉSULTATS :
Au total, 367 cas d’ITCM ont été diagnostiqués pendant la période de l’étude. Quarante-cinq patients (12,3 %) avaient des lésions qui avaient fait l’objet d’une analyse de culture et de sensibilité. Le S aureus était l’organisme le plus observé, 14 cas (66 %) étant sensibles à la méthicilline et sept (33 %) étant résistants à la méthicilline. Dans les sept cas de SARM, un seul patient présentait des facteurs de risque évidents de SARM d’origine nosocomiale. Des céphalosporines de première génération avaient d’abord été prescrites à 280 patients (76 %).
CONCLUSIONS :
L’incidence globale de SARM au sein de la population qui consulte à une salle d’urgence pédiatrique de Terre-Neuve-et-Labrador semble faible, même si seulement un petit pourcentage de ces infections a fait l’objet d’une culture. À l’heure actuelle, il ne semble pas nécessaire de modifier la couverture antibiotique empirique, soit une céphalosporine de première génération.
Methicillin-resistant Staphylococcus aureus (MRSA) first emerged in the 1960s, soon after the introduction of the antistaphylococcal antibiotics (1). Initially, it was observed only as a hospital-acquired MRSA (HA-MRSA) infection (2,3). Beginning in the late 1980s, there were increasing reports of MRSA occurring in previously healthy patients who had no contact with the health care system (4,5). Community-associated MRSA (CA-MRSA) has different phenotypic and genetic characteristics than HA-MRSA. Risk factors for hospital-acquired infection include prolonged hospitalization, dialysis, indwelling catheters, admission to an intensive care unit, previous antibiotic therapy and surgical procedures. HA-MRSA is usually associated with pneumonia, urinary tract, bloodstream or surgical-site infections (6). CA-MRSA tends to cause infection in healthy, young individuals, and overwhelmingly causes skin and soft tissue infections (SSTIs) (1,6). Without proper treatment, these infections may progress to severe infections involving skin, muscle or bone (7–9). CA-MRSA can also cause severe illnesses such as necrotizing fasciitis, severe sepsis, and pneumonia with respiratory failure and death, especially in pediatric patients (5,9,10,11).
The typical agents causing SSTIs are Gram-positive organisms such as methicillin-sensitive S aureus and Streptococcus pyogenes. In the past, cultures were not routinely obtained because of the predictable etiology (5). Clinicians usually rely on the local prescribing guidelines for choosing appropriate antibiotic therapy. These guidelines need to reflect the risk of an infection being caused by MRSA (12). First-generation cephalosporins and antistaphylococcal penicillins are typically the first choice for SSTIs, meaning that many MRSA infections may not be initially properly treated (2). Because some strains of CA-MRSA are extremely virulent, it is not recommended to withhold appropriate antibiotic treatment in unwell individuals when CA-MRSA may be the causative organism (12). Some hospitals have changed their recommendations for first-line empirical treatment of SSTIs based on the high prevalence of MRSA in their community (13,14). In any case, it is now important that cultures of SSTIs are obtained to monitor CA-MRSA in the community as well as guide appropriate antibiotic therapy (14). Because of the differences in the treatment of the infections, it is important for clinicians to be aware of the incidence of HA-MRSA and CA-MRSA in their communities to make appropriate first-line treatment choices.
CA-MRSA is not a reportable disease in Canada; while some provinces track infection rates (1), the rate of incidence is unknown for many parts of the country. There is evidence that CA-MRSA rates vary across regions and that it may have higher incidence rates than HA-MRSA in some areas. In patients presenting to an emergency department in California (USA) with an SSTI, MRSA was present in almost 50% of infections. Seventy-five per cent of those infections were classified as CA-MRSA (14). A study at a large hospital in Georgia (USA) showed that 72% of SSTIs were caused by MRSA, and 63% of those were community associated (15). There is also evidence that rates of CA-MRSA are increasing in some regions. For example, a surveillance study in Minnesota (USA) found that the number of MRSA cases classified as community associated increased from 11% to 33% in six years (16).
In the present study, we examined the rates of HA-MRSA and CA-MRSA identified in SSTIs in Newfoundland and Labrador, a province for which little is known about the rates of MRSA, particularly in the pediatric population. The Newfoundland Department of Health and Community Services releases quarterly reports on the incidence of MRSA in the various health regions; however, these reports do not specify patient age nor type of infection. The emergency department is a key place in a health care facility for first identifying infections and is likely to encounter the most cases of CA-MRSA. Because of the possible severity of infections in children, we focused on identifying rates at the only pediatric emergency department in Newfoundland and Labrador. The Janeway Children’s Health and Rehabilitation Centre (St John’s, Newfoundland and Labrador) is the only tertiary care children’s hospital in Newfoundland and Labrador. It has approximately 30,000 patient visits to its emergency department each year (17). As the province’s only pediatric tertiary care centre, its emergency department sees patients from all parts of the province, although the majority are from the St John’s area. We examined the incidence of both CA-MRSA and HA-MRSA in patients presenting to the Janeway Emergency Department with SSTIs. Specifically, we sought to determine the organisms responsible for infection and the antibiotic used for treatment.
METHODS
The records for all visits to the Janeway Emergency Department were reviewed using data recorded by emergency department staff for the period between April 15, 2010 and April 14, 2011. The discharge diagnosis as recorded on the emergency room chart was used to identify all patients diagnosed with cellulitis, abscess, folliculitis, impetigo and skin infection (not otherwise specified). The medical records were reviewed in detail to determine whether a culture had been obtained, the results of the culture and the antibiotic that had been prescribed. The diagnosis of periorbital cellulitis was excluded; it would be very rare for a periorbital cellulitis to have a lesion amenable to culturing.
Staphylococcus was identified in the microbiology laboratory based on Gram reaction and shape, and confirmed using catalase and latex agglutination for clumping factor, protein A and capsular polysaccharides (all assessed using Pastorex Staph-Plus [Biorad, USA]). Identification and susceptibility was performed using a MicroScan GP34 panel (Siemens, Germany), and discrepancies or questionable susceptibility were confirmed using Kirby Bauer testing. If a patient was found to have MRSA, risk factors for CA-MRSA were assessed using the clinical criteria for the definition of CA-MRSA according to the Centers for Disease Control and Prevention (Georgia, USA) (18). The research protocol was approved by the Human Investigation Committee at Memorial University (St John’s, Newfoundland and Labrador).
RESULTS
Records were reviewed for all patient visits occurring between April 15, 2010 and April 14, 2011, except for the month of February 2011. These records could not be located and, therefore, were not reviewed. A total of 367 patients were identified as having a SSTI during the 11 months for which data were reviewed. There were 167 cases of cellulitis, 82 cases labelled as skin infection (not otherwise specified) and 45 cases of abscess. Impetigo was identified in 33 patients, and 40 patients had diagnoses such as folliculitis and infected ingrown toenail (classified as ‘other’) (Figure 1). Forty-five patients (12.3%) had lesions that were swabbed, and sent for culture and sensitivity analysis. Nine cases of cellulitis (5.4%), nine skin infections (11%), 26 abscesses (57.8%) and one case of impetigo (3.0%) were cultured. No infection identified as ‘other’ was cultured.
Figure 1).
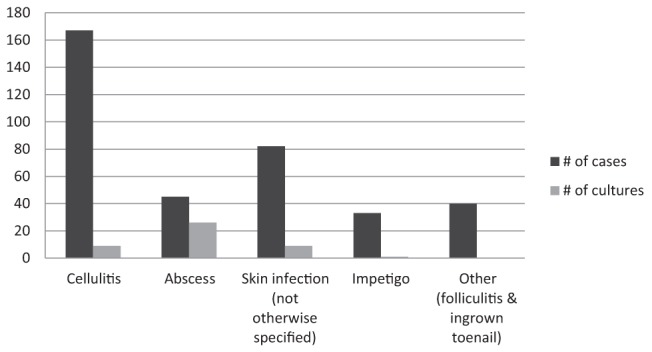
Number of cases of skin and soft tissue infection identified and the number of cultures taken between April 2010 and April 2011 according to type of infection
Of the 45 cases for which cultures were taken, the causative organism was S aureus in 21 (46%). Coagulase-negative Staphylococcus was identified in nine patients (20%) and no growth was identified in seven patients (16%) despite having their lesion swabbed (Figure 2). Other organisms that were identified less frequently included coliforms in five cases, group A streptococcus in one case and anaerobic bacteria in two cases. Multiple organisms were identified in seven cases. Fourteen cases (66%) in which S aureus was isolated were methicillin sensitive, and seven (33%) were methicillin resistant.
Figure 2).
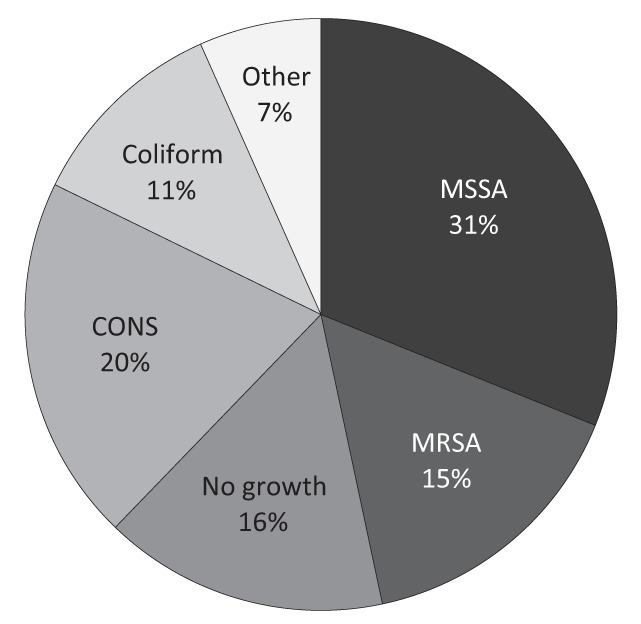
Etiological organism for cultured skin and soft tissue infections. MSSA Methicillin-sensitive Staphylococcus aureus; MRSA Methicillin-resistant S aureus; CONS Coagulase-negative Staphylococcus; Other Group A Streptococcus, anaerobic bacteria
Of the seven cases of MRSA identified, one case was in a patient who had undergone open heart surgery at a hospital outside the province. Another patient was from Labrador. The other five patients lived in and around the St John’s area and had no identifiable risk factors for a hospital-acquired infection on review of their medical records. The antibiotic susceptibility patterns for all seven patients were the same. The isolates were universally sensitive to clindamycin, trimethoprim-sulfamethoxazole (TMP-SMZ) and vancomycin, and universally resistant to erythromycin, amoxicillin-clavulanic acid and the first-generation cephalosporins. First-generation cephalosporins were overwhelmingly the antibiotic of choice for the treatment of SSTIs, being selected nearly 75% of the time (Figure 3). Other commonly prescribed antibiotics included third-generation cephalosporins (ceftriaxone in 3.8% of cases and cefotaxime in 1.3%), clindamycin (4%), and cloxacillin (2%) and amoxicillin-clavulanic acid (2%). Topical antibiotics were used in 4.9% of cases.
Figure 3).
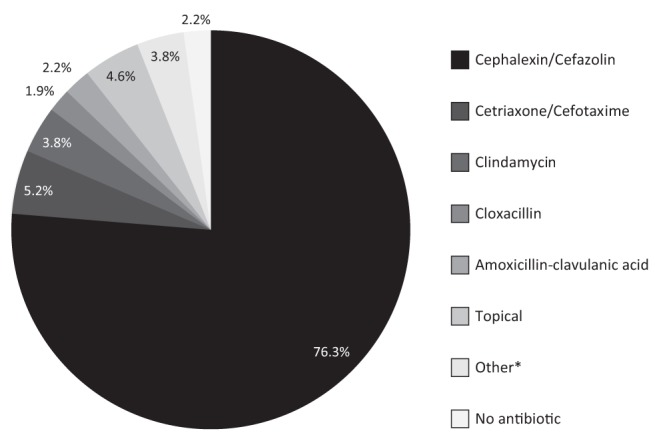
First-line treatment of skin and soft tissue infections. *Other includes metronidazole (1%), cefuroxime (1%), erythromycin (1.9%), trimethoprim-sulfamethoxazole, amoxicillin, ampicillin and gentamycin (all <1%)
DISCUSSION
To the best of our knowledge, the present study is the first to report on pediatric MRSA in Newfoundland and Labrador. There were 367 cases of skin infection identified during the 11 months between April 15, 2010 and April 14, 2011. Of these, 12.3% had samples obtained for culture and sensitivity. While some infections were unlikely to be amenable to culture, all abscesses should undergo incision and drainage, and lesions with discharge, especially those that do not respond to initial treatment, should be cultured (19). The culture rate of 12.3% appears to be lower compared with other studies that have investigated SSTIs, in which rates have ranged from 17% to as high as 50% to 100% (20–22). It is unclear why the culture rate in the present study was lower.
The incidence of MRSA during the 11 months studied was <2% of all skin infections. However, in infections for which cultures had been taken, MRSA accounted for 15.6% of the organisms and 33% of S aureus isolates. In only one instance was there a clear link to a hospital-acquired infection. Uncomplicated skin abscesses in children >3 months of age can be managed by incision and drainage alone, coupled with close follow-up (19). Guidelines published in 2006 suggested that when more than 10% to 15% of the S aureus isolates are MRSA, empirical therapy for infections should include coverage for MRSA (23). Because microbiological diagnosis was obtained in only a few cases, it is difficult to determine the true rate of MRSA infection in children in the province; however, based on the data available, it appears that we may be reaching this threshold. Further surveillance is, therefore, warranted.
All MRSA isolates exhibited the same sensitivity profile and were sensitive to clindamycin, TMP-SMZ and vancomycin. In general, CA-MRSA tends to display the same broad sensitivity patterns, while HA-MRSA tends to be resistant to nearly all antibiotics except vancomycin and other antibiotics not tested here (eg, linezolid). Review of medical records also revealed only one patient with clear risk factors for HA-MRSA, having undergone open heart surgery in another province. The rest of the cases appeared to occur in otherwise healthy individuals, indicating that MRSA is in the community.
The present study had a number of limitations. One of the key barriers to the study was that the required information regarding emergency visits was only available in paper records. This format of storing patient records made the review very time consuming and likely limits the ability to easily identify changes in the infection patterns of SSTI in the pediatric population. It also increases the risk of loss of patient information. The paper record for the month of February 2011 was misplaced and could not be located to be reviewed for the present study. In the months immediately preceding and following February, approximately 20 skin infections were identified and an average of five swabs taken. It is not possible to speculate on whether any of the skin infections in February may have been caused by MRSA. Another weakness was relying on the discharge diagnoses from the emergency room documentation to identify cases. In some instances, there was no diagnosis listed, or it was unclear what the diagnosis was. In these cases, every attempt was made to identify the hospital chart number and verify the visit, but there may have been some cases that were missed. Having accurate electronic records would have helped to avoid some of these issues. The study did not attempt to determine the levels of infection identified in other centres across the province and it is not known how many cases would have been missed. The finding that infections are swabbed at such a low rate also hampered the determination of more accurate rates of MRSA in the pediatric population in the province.
The present study found that while the overall prevalence of MRSA in patients sampled was low, when microbiological diagnosis was obtained, cases of what appear to be CA-MRSA exist. The incidence of CA-MRSA is currently too low to recommend changing the first-line antibiotic treatment, which is a first-generation cephalosporin. Follow-up needs to be assured, and for infections that do not appear to be responding to first-line treatment, MRSA should be considered in the differential diagnosis. Antibiotic coverage should be changed to account for this; options may include TMP-SMZ or clindamycin. The study also highlighted the low number of cultures taken from skin lesions and the need to review whether taking a greater percentage of cultures from SSTIs would be appropriate. Finally, while the percentage of MRSA infections identified in the SSTIs is low, the higher percentage of MRSA infections in the SSTIs for which cultures were taken supports the conclusion that further surveillance of the incidence of the CA-MRSA in the pediatric population in Newfoundland and Labrador is warranted.
Footnotes
DISCLOSURES: This work originated in the Division of Pediatrics, Faculty of Medicine, Memorial University of Newfoundland and the Janeway Children’s Health and Rehabilitation Centre (St John’s, Newfoundland and Labrador).
REFERENCES
- 1.Wallin TR, Hern HG, Frazee BW. Community-associated methicillin-resistant Staphylococcus aureus. Emerg Med Clin N Am. 2008;26:431–55. doi: 10.1016/j.emc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Jungk J, Como-Sabetti K, Stinchfield P, et al. Epidemiology of methicillin-resistant Staphylococcus aureus at a pediatric healthcare system, 1991–2003. Pediatr Infect Dis J. 2007;26:339–44. doi: 10.1097/01.inf.0000257452.58182.e1. [DOI] [PubMed] [Google Scholar]
- 3.Whitman TJ. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Dis Mon. 2008;54:780–6. doi: 10.1016/j.disamonth.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Taylor G, Kirkland T, Kowalewska-Grochowska K, et al. A multistrain cluster of methicillin-resistant Staphylococcus aureus based in a Native community. Can J Infect Dis. 1990;1:121–6. doi: 10.1155/1990/618630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moran GJ, Amii RN, Abrahamian FF, et al. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infect Dis. 2005;11:928–30. doi: 10.3201/eid1106.040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavez TT, Decker CF. Health care-associated MRSA versus community-associated MRSA. Dis Mon. 2008;54:763–8. doi: 10.1016/j.disamonth.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Miller LG, Kaplan SL. Staphylococcus aureus: A community pathogen. Infect Dis Clin N Am. 2009;23:35–52. doi: 10.1016/j.idc.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 8.McCaig LF, McDonald LC, Mandal S, et al. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis. 2006;12:1715–23. doi: 10.3201/eid1211.060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus – Minnesota and North Dakota, 1997–1999. MMWR. 1999;48:707–10. [PubMed] [Google Scholar]
- 11.Gonzalez BE, Martinez-Aguilar G, Hulten KG, et al. Severe staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005;115:642–8. doi: 10.1542/peds.2004-2300. [DOI] [PubMed] [Google Scholar]
- 12.Eady AE, Cove JH. Staphylococcal resistance revisited: Community-acquired methicillin-resistant Staphylococcus aureus – an emerging problem for the management of skin and soft tissue infections. Curr Opin Infect Dis. 2003;16:103–24. doi: 10.1097/00001432-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Hussain FM, Boyle-Vavra S, Bethel CD, et al. Current trends in community-acquired methicillin-resistant Staphylococcus aureus at a tertiary care pediatric facility. Pediatr Infect Dis J. 2000;19:1163–6. doi: 10.1097/00006454-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Frazee BW, Lynn J, Charlebois ED. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med. 2005;45:311–20. doi: 10.1016/j.annemergmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 15.King MD, Humphrey BJ, Wang YF, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144:309–17. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 16.Como-Sabetti K, Harriman KH, Buck JM, et al. Community-associated methicillin-resistant Staphylococcus aureus: Trends in case and isolate characteristics from six years of prospective surveillance. Public Health Rep. 2009;124:427–35. doi: 10.1177/003335490912400312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janeway Foundation website. < www.janewayfoundation.nf.ca/AboutTheJaneway/> (Accessed March 1, 2012).
- 18.Community-associated MRSA information for clinicians. < www.cdc.gov/mrsa/diagnosis/> (Accessed March 1, 2012).
- 19.Robinson JL, Salvadori MI. Management of community-associated methicillin-resistant Staphylococcus aureus skin abscesses in children. Paediatr Child Health. 2011;16:115–6. doi: 10.1093/pch/16.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a US population: A retrospective population-based study. BMC Infect Dis. 2013;13:252. doi: 10.1186/1471-2334-13-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parnes B, Fernald D, Coombs L, et al. Improving the management of skin and soft tissue infections in primary care: A report from state networks of Colorado ambulatory practices and partners (SNOCAP-USA) and distributed ambulatory research in therapeutics network (DARTNet) J Am Board Fam Med. 2011;24:534–42. doi: 10.3122/jabfm.2011.05.110018. [DOI] [PubMed] [Google Scholar]
- 22.Karamatsu ML, Thorp AW, Brown L. Changes in community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections presenting to the pediatric emergency department. Pediatr Emerg Care. 2012;28:131. doi: 10.1097/PEC.0b013e318243fa36. [DOI] [PubMed] [Google Scholar]
- 23.Barton M, Hawkes M, Moore D, et al. Guidelines for the prevention and management of community-associated methicillin-resistant Staphylococcus aureus: A perspective for Canadian health care practitioners. Can J Infect Dis Med Microbiol. 2006;17(Suppl C):4C–24C. [PMC free article] [PubMed] [Google Scholar]


