Abstract
Adaptive radiotherapy (ART) has recently been introduced to restore the planned dose distribution by accounting for the anatomic changes during treatment. By quantifying the anatomic changes in nasopharyngeal carcinoma (NPC) patients, this study aimed to establish an ART strategy for NPC cases. A total of 30 NPC patients treated with helical tomotherapy were recruited. In the pretreatment megavoltage CT images, the anatomic changes of the posterolateral wall of nasopharynx (P-NP), neck region and parotid glands were measured and assessed. One-way repeated measure ANOVA was employed to define threshold(s) at any time-point. The presence of a threshold(s) would indicate significant anatomical change(s) such that replanning should be suggested. A pragmatic schedule for ART was established by evaluating the threshold for each parameter. Results showed the P-NP, parotid gland and neck volumes demonstrated significant regressions over time. Respectively, the mean loss rates were 0.99, 1.35, and 0.39 %/day, and the mean volume losses were 35.70, 47.54 and 11.91% (all P < 0.001). The parotid gland shifted medially and superiorly over time by a mean of 0.34 and 0.24 cm, respectively (all P < 0.001). The neck region showed non-rigid posterior displacement, which increased from upper to lower neck. According to the threshold occurrences, three replans at 9th, 19th and 29th fractions were proposed. This ART strategy was able to accommodate the dosimetric consequences due to anatomic deviation over the treatment course. It is clinically feasible and would be recommended for centers where an adaptive planning system was not yet available.
Keywords: adaptive radiotherapy, nasopharyngeal carcinoma, tomotherapy, replanning schedule
INTRODUCTION
Intensity-modulated radiotherapy with image-guidance technique (IG-IMRT) is commonly used in treating head and neck (HN) cancer nowadays. Daily image guidance using computed tomography (CT) such as cone-beam (CB) CT [1–3] and megavoltage (MV) CT [4] provide volumetric images for treatment verification. This allows precise set-up error correction prior to each fraction of treatment. Nevertheless, HN cancer patients usually experience non-rigid anatomic changes such as radiation-induced tumor/parotid shrinkage and weight loss throughout the prolonged radiotherapy course (6–8 weeks) [5–9]. These changes could not be corrected by simple couch shifting and could lead to violation of the original treatment plan that results in poor target coverage and/or overdosing to surrounding organs-at-risk (OARs). The potential consequences would be decreased tumor control and/or severe complications [10–13]. Adaptive radiotherapy (ART) has recently been introduced, in which the treatment plan is modified according to the tumor response and organ deformations during a radiotherapy course. By restoring the initial planned dose distribution, ART minimizes the unintended normal tissue toxicity and maintains the tumor coverage.
ART on a daily basis, i.e. to perform plan modification daily, could heavily increase the department workloads as CT scanning, contouring and plan optimization would need to be repeated every day. Organ changes in HN cases were shown to follow a progressive trend rather than random changes [5–7]; it is therefore possible to deploy ART on an interval basis i.e. only when significant changes are observed. This approach potentially reduces the need for related overheads, time and manpower with improved dosimetric results. Various studies in HN cases have demonstrated the dosimetric benefits of replanning once at the mid-course of treatment. Hansen et al. [14] repeated CT scanning after 19 (mean ± 6) fractions of treatment for replanning purposes and showed improvement in target coverage and OAR dose reduction. Kuo et al. [9] carried out replanning after 25 fractions for patients with enlarged neck lymph nodes and showed that replanning according to the parotid medial shift caused by lymph node regression (>50%) could provide the dosimetric benefit of more than 3 Gy reduction in the mean dose. Wang et al. [15] conducted a similar study on nasopharyngeal carcinoma (NPC) patients. They showed that although the target coverage was maintained with more high-dose region presence in targets, the doses to various OARs increased significantly (0.05–7.8 Gy) if no replanning was performed after 18 fractions of treatment. Consistent results were found in the study of Kim et al. [16]. They also reported insignificant dose changes to targets but significantly higher doses to parotids, brainstem and spinal cord without replanning. Previously, our team demonstrated that a three-phase adaptive approach in NPC gave significant improvement in sparing the OARs while keeping satisfactory target dose coverage [17]. Although this study revealed that multiple replannings were deemed necessary for NPC, there is as yet no consensus on when and how often the replanning should be performed. Reports on defining ART strategy are lacking. In our center, most NPC cases are treated with helical tomotherapy and daily megavoltage computed tomography (MVCT) is routinely performed for on-line treatment verification. These images provide valuable anatomic information on the progressive organ changes over time. Using the MVCT images, this study first quantified and characterized the volumetric and geometric changes of tumors and OARs in NPC patients. An ART strategy for NPC was then established by defining various thresholds that indicated significant anatomic changes and hence the need for replanning.
MATERIALS AND METHODS
Patient characteristics
We recruited a total of 30 NPC cases treated with Hi-Art tomotherapy (TomoTherapy Inc., Madison, WI) in 2006–2008. All patients received a three-phase radiotherapy protocol with a total of 37–38 fractions, in which there were 25 or 30 fractions in phase one (PI), 5–10 fractions in phase two (PII) and 2–4 fractions in phase three (PIII). Repeat CT scanning and replanning were performed for PII and PIII. Table 1 presents the patient characteristics for this study. All patients recruited were newly diagnosed with NPC without distant metastasis at the time of treatment. The majority (50%) of patients suffered from Stage III disease. Histologically, all except one patient were diagnosed as having undifferentiated carcinomas. Concurrent chemotherapy was given to those patients with Stage II to Stage IV disease. In particular, 73% and 27% of patients received a total of 38 and 37 fractions of treatment, respectively.
Table 1.
Patient and disease characteristics
| No. of patients | % | |
|---|---|---|
| Gender | ||
| Male | 23 | 77% |
| Female | 7 | 23% |
| Tumor stage | ||
| T1 | 9 | 30% |
| T2b | 16 | 53% |
| T4 | 5 | 17% |
| Nodal stage | ||
| N0 | 7 | 23% |
| N1 | 5 | 17% |
| N2 | 16 | 53% |
| N3a | 1 | 3% |
| N3b | 1 | 3% |
| Stage group | ||
| I | 3 | 10% |
| IIB | 7 | 23% |
| III | 15 | 50% |
| IVA | 3 | 10% |
| IVB | 2 | 7% |
| Histology | ||
| Undifferentiated | 29 | 97% |
| SCC | 1 | 3% |
| Concurrent chemotherapy | ||
| Yes | 27 | 90% |
| No | 3 | 10% |
| 6 fractions/week | ||
| Yes | 20 | 67% |
| No | 10 | 33% |
| Total no. of fractions | ||
| 37 | 8 | 27% |
| 38 | 22 | 73% |
Treatment planning and delivery
All patients were immobilized with T-VacLok and thermocast (Med-Tec Inc., Orange City, IA) under the HN region during CT simulations and treatment. The same oncologist was responsible for all target volume delineation for plan generation. The prescribed dose to primary targets was 79.0–84.6 Gy (mean: 82.6 Gy). During plan optimization, effort had been put into keeping the critical organ doses within acceptable tolerance. The dose constraint for parotid gland was primarily set as a mean dose <26 Gy [18–20], but this was difficult to achieve due to the close proximity of parotid glands to target volumes and the concerns about sparing other more critical organs (brainstem, spinal cord and optical organs). The planned mean dose was 35.4 Gy on average. Prior to each tomotherapy treatment, MVCT with 6-mm thickness was taken and registered to the corresponding planning CT for verification of patient set-up accuracy. The scan range was set to just encompass the target volumes and the bilateral parotid glands in order to minimize radiation dose to patient and patient on couch time. These MVCT images were exported to the Eclipse Treatment Planning System (TPS) (Varian Medical Systems, Palo Alto, CA) for geometric analysis of the selected structures.
Volumetric and geometric analysis
Contouring was done using the Eclipse TPS on alternate-day MVCTs starting from the first fraction. Altogether there were 19 MVCT image sets for each patient, which gave a total of 570 sets in the study. On each MVCT set, the posterolateral wall of nasopharynx (P-NP), bilateral parotid glands and neck volume covering the whole cervical spine level were manually contoured using consistent window and level settings. The P-NP basically included the soft tissues at the posterior pharyngeal wall, prevertebral muscle, fossa of Rosenmüller, torus tubarius, opening of the Eustachian tube, and the tensor and levator veli palatini. Since NPC frequently arises from the lateral and the superoposterior walls of nasopharynx [21, 22], the anatomic changes in the P-NP could directly reflect the changes of the gross tumor. Besides, the vertebral canals at the levels of 3 cm (VC3), 6 cm (VC6) and 9 cm (VC9) inferior to the base of skull (BOS) were also outlined. The geometric changes of these three slices of contours should demonstrate changes in the upper, middle and lower portion of the cervical cord as well as the corresponding neck regions. To eliminate the interobserver variance, the same oncologist was responsible for the contouring of the P-NP and the same radiation therapist performed all the normal structure delineation in each MVCT set for all patients.
For each patient, the volumes of the P-NP, bilateral parotid glands and neck were calculated by the TPS and recorded. The positions of the P-NP, bilateral parotid glands, VC3, VC6 and VC9 were monitored throughout the treatment course. In each MVCT, a reference point (0, 0, 0) was defined at the BOS level at the midsagittal plane. As BOS is a rigid bony landmark, this point was easily reproducible to correlate the whole series of MVCT sets and therefore served as a good reference. The center of each structure mass (COM), which was expressed in positional vectors (x, y, z) relative to the reference point, was then calculated by the TPS. In this way, the structure displacements in lateral (x), longitudinal (y) and vertical (z) directions were tracked over time. For VC3, VC6 and VC9, the evaluation of the longitudinal displacement was excluded. Graphs with trend lines were plotted to visualize the specific patterns of anatomic changes over time. A paired t-test was applied to compare the result before and after the treatment course; linear regression was used to quantify their rates of changes; and a Pearson correlation test was employed to investigate the association of the pretreatment parameters with the anatomic changes during treatment.
Defining ART strategy for NPC cases
During volumetric and geometric analysis, any parameters that showed progressive anatomic changes were under consideration for setting the ART strategy in NPC cases. One-way repeated measure analysis of variance (ANOVA) was applied to indicate the statistically significant anatomic changes (thresholds) at any time-point of the treatment course such that replannings should be suggested. To begin with, the data in the first treatment fraction was compared with those in subsequent fractions separately until the result reached statistical difference. For example, if significant difference (i.e. significant anatomic change) was seen between the data in the 9th and the first fraction, the 9th fraction would be the first threshold point at which replanning was assumed. The data in the 9th fraction was then compared with those in subsequent fractions in order to define the next threshold point, and so on. In this way, the threshold points for each selected parameter throughout the treatment course could be identified. A pragmatic schedule for ART was ultimately established by accounting for all thresholds appearing in all parameters in terms of efficacy and feasibility.
RESULTS
Volumetric and geometric analysis
P-NP
Progressive P-NP volume reduction was observed in all patients throughout the radiotherapy course. The P-NP tended to lose volume faster in the first half of the treatment course (Fig. 1). At the end of treatment, the mean P-NP volume dropped significantly by 35.70 ± 20.06% (P < 0.001). Using linear regression, the mean volume loss rate was 0.99 ± 0.55%/day. There were no significant changes in P-NP geometric location, with its mean COM position close to zero throughout the course (Fig. 2). There was a mild negative correlation between the initial P-NP volume and its percentage volume loss at the end of the treatment course (correlation coefficient = − 0.452; P = 0.014; Fig. 3).
Fig. 1.
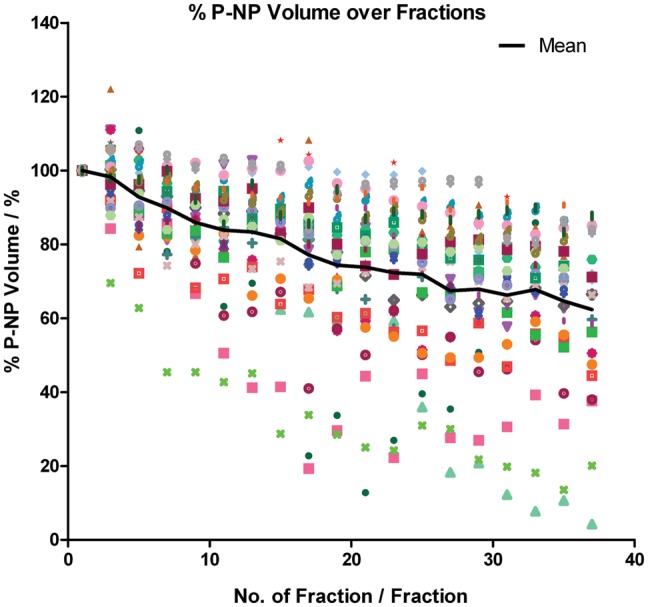
Relative P-NP volume over the treatment course.
Fig. 2.
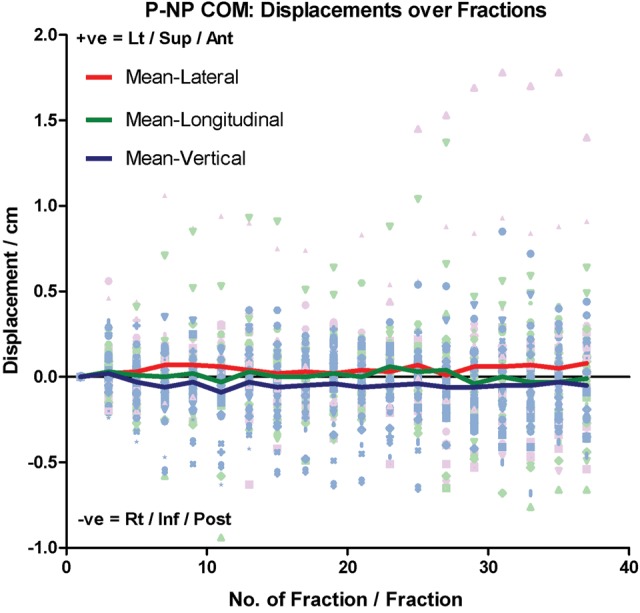
Relative COM displacement of P-NP in lateral, longitudinal and vertical direction over the treatment course. Lt = Left, Sup = Superior, Ant = Anterior, Rt = Right, Inf = Inferior and Post = Posterior.
Fig. 3.
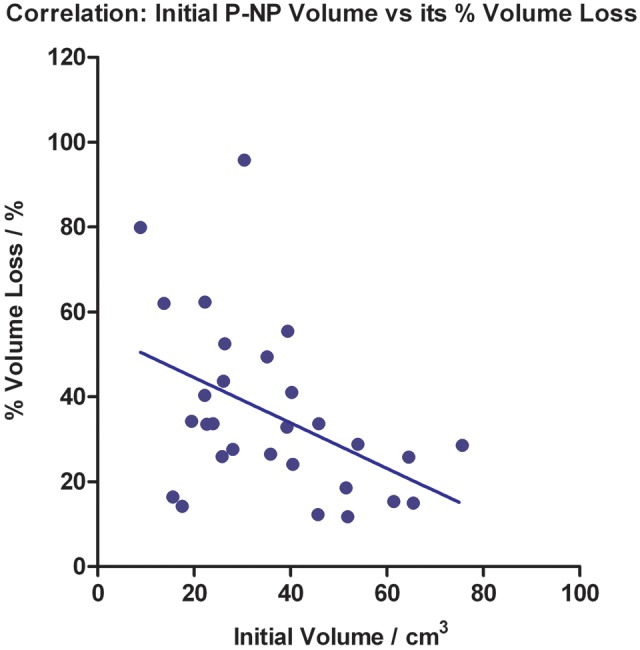
Correlation between the initial P-NP volume and its percentage volume loss at the end of the treatment course (Linear fit displayed).
Parotid glands
All parotid volumes demonstrated similar reduction trends over time. The mean percentage volume loss was 47.54 ± 14.27% (P < 0.001) at treatment completion, and the mean loss rate was estimated to be 1.35 ± 0.39%/day (Fig. 4). The mean COM of the parotid gland shifted progressively towards the medial and superior aspects during treatment, and remained fairly constant when close to the treatment end (Fig. 5). The mean medial and superior displacements were 0.34 ± 0.27 cm and 0.24 ± 0.39 cm, respectively (both P < 0.001), and the mean shift rate was 0.01 ± 0.01 cm/day in both directions. There was no specific trend for the mean parotid COM displacement in the vertical direction (Fig. 5). No significant correlation was found between the initial parotid volume and its percentage volume loss at the end of the treatment course (correlation coefficient = − 0.118; P = 0.368; Fig. 6).
Fig. 4.
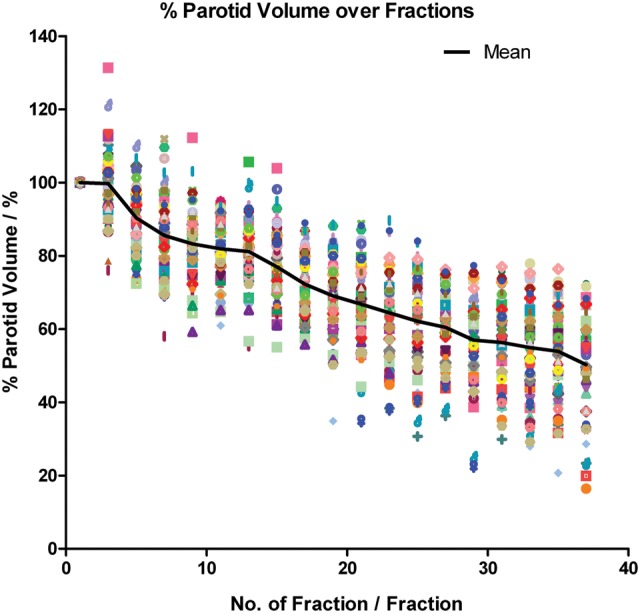
Relative parotid volume over the treatment course.
Fig. 5.
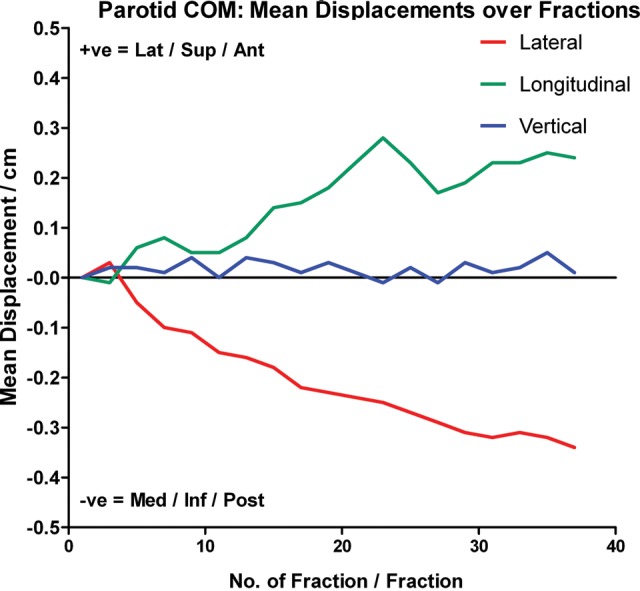
Mean COM displacement of parotid gland in lateral, longitudinal and vertical direction over the treatment course. Lat = Lateral, Sup = Superior, Ant = Anterior, Med = Medial, Inf = Inferior and Post = Posterior.
Fig. 6.
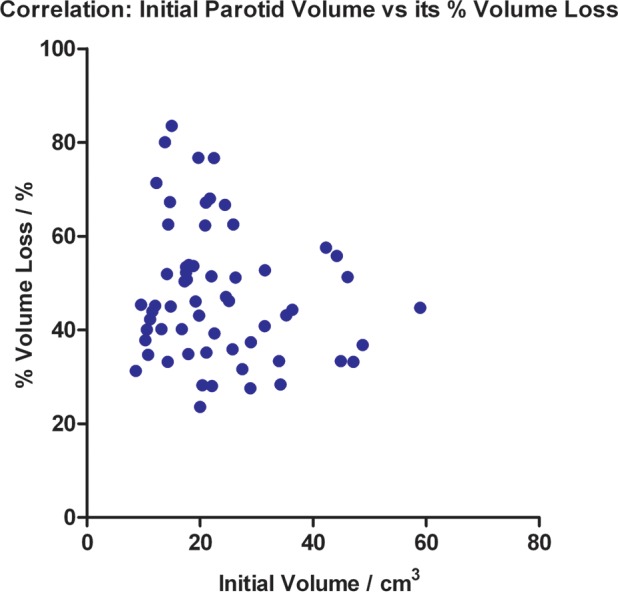
Correlation between the initial parotid volume and its percentage volume loss at the end of the treatment course.
Neck volume
All patients experienced changes of habitus in the neck region, manifested by a progressive reduction trend in the mean neck volume throughout the treatment course. The neck volume dropped slightly faster in the first half of the course (Fig. 7). The mean reduction was 11.91 ± 5.57% (P < 0.001) towards the end of treatment and the mean loss rate was 0.39 ± 0.15%/day.
Fig. 7.
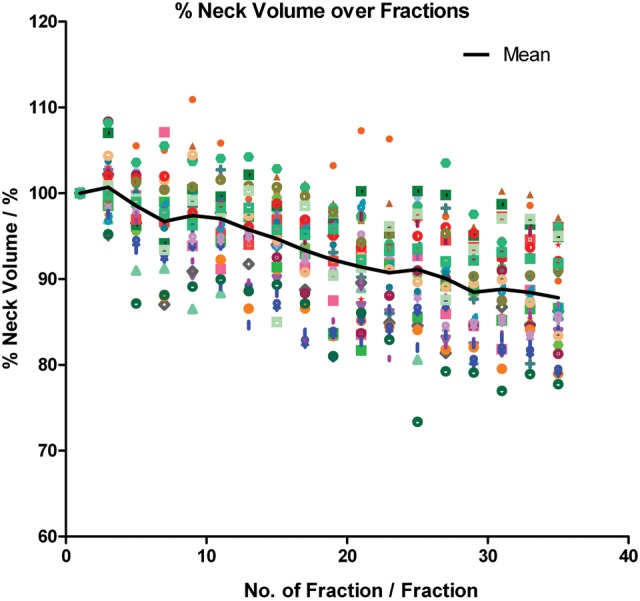
Relative neck volume over the treatment course.
COM positions of VC3, VC6 and VC9
There were no significant changes in the mean COMs of VC3, VC6 and VC9 in the lateral aspect during treatment. Nevertheless, the mean COMs of all VCs shifted significantly to the posterior direction (Fig. 8). Progressive downward trends were noted in all these vertebral levels, with the mean COM displacements increasing with time, especially after two-thirds of the course. Moreover, the magnitude of the mean COM shift also increased from VC3 towards VC9, which implied that there was a greater variation in lower neck position throughout the treatment course. At treatment completion, the mean posterior displacements of the VC3, VC6 and VC9 COMs were 0.04 ± 0.22 cm (P = 0.221), 0.18 ± 0.31 cm (P = 0.006) and 0.24 ± 0.36 cm (P = 0.001), respectively.
Fig. 8.
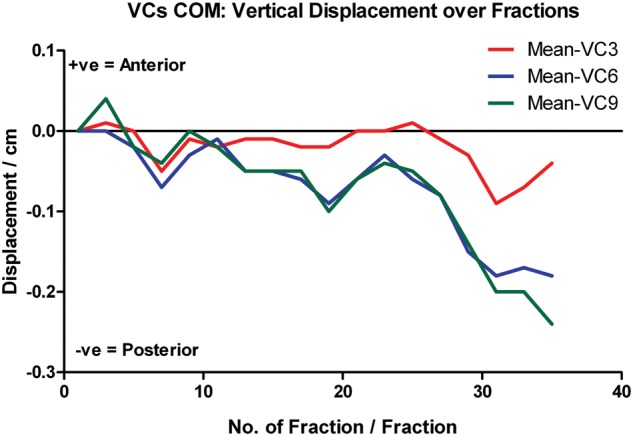
Relative COM displacement of VC3, VC6 and VC9 in vertical direction over the treatment course.
ART strategy for NPC cases
From the volumetric and geometric analysis, the P-NP, parotid and neck volumes, the medial and superior displacement of the parotid COM, and the posterior displacement of COMs of VC6 and VC9 demonstrated significant progressive changes. These parameters were under consideration for establishing the ART strategy in NPC cases. Table 2 lists the threshold occurrences in these parameters throughout the treatment course based on the ANOVA results. Each threshold represented a statistically significant anatomic change such that replanning was preferred. The frequency of replanning (threshold occurrence) varied between different parameters. In practical consideration, three replans at the 9th, 19th and 29th fractions were proposed, as most of the thresholds appeared in these time-points. Table 3 summarizes the corresponding degrees of anatomic changes at the proposed replanning fractions.
Table 2.
Replanning schedules for selected parameters
| Week | Treatment fraction | P-NP % volume | Parotid % volume | Parotid COM medial shift | Parotid COM superior shift | Neck % volume | VC6 COM posterior shift | VC9 COM posterior shift | Total no. of thresholds |
|---|---|---|---|---|---|---|---|---|---|
| Week 1 | 1st | 0 | |||||||
| 3rd | 0 | ||||||||
| 5th | a | 1 | |||||||
| Week 2 | 7th | a | 1 | ||||||
| 9th | a | a | 2 | ||||||
| Week 3 | 11th | 0 | |||||||
| 13th | a | 1 | |||||||
| 15th | a | 1 | |||||||
| Week 4 | 17th | a | 1 | ||||||
| 19th | a | a | a | 3 | |||||
| Week 5 | 21st | a | 1 | ||||||
| 23rd | 0 | ||||||||
| 25th | a | 1 | |||||||
| Week 6 | 27th | 0 | |||||||
| 29th | a | a | a | 3 | |||||
| Week 7 | 31st | a | 1 | ||||||
| 33rd | 0 | ||||||||
| 35th | a | 1 | |||||||
| Week 8 | 37th | 0 |
aIndicates threshold occurrence on a particular fraction of treatment, in which replanning is suggested.
Table 3.
Degrees of anatomic changes (mean) of selected parameters at the proposed replanning fractions
| Treatment fractions |
|||
|---|---|---|---|
| 9th | 19th | 29th | |
| P-NP % volume loss | 14.05 % | 25.64 % | 32.11 % |
| Parotid % volume loss | 16.70 % | 30.94 % | 43.03 % |
| Parotid COM medial shift | 0.11 cm | 0.23 cm | 0.31 cm |
| Parotid COM superior shift | 0.05 cm | 0.18 cm | 0.19 cm |
| Neck % volume loss | 2.58 % | 7.76 % | 11.54 % |
| VC6 COM posterior shift | 0.03 cm | 0.09 cm | 0.15 cm |
| VC9 COM posterior shift | 0.00 cm | 0.10 cm | 0.14 cm |
DISCUSSION
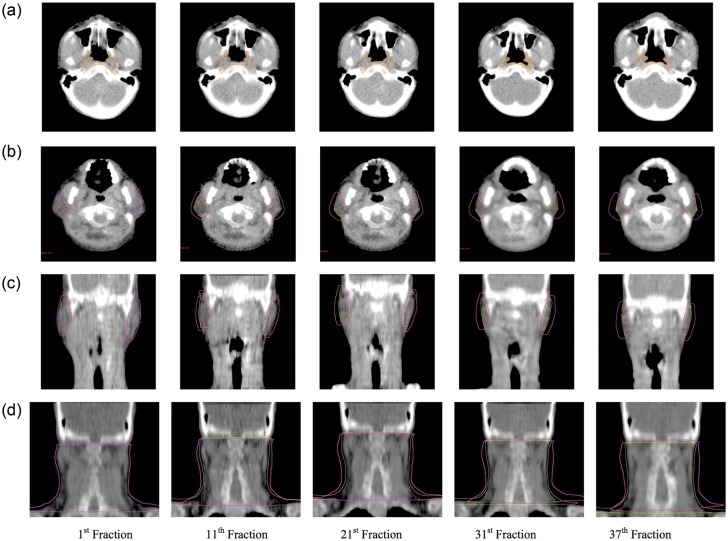
ART in NPC cases prevents excessive dose to OARs while maintaining the target dose, improving the dosimetric and hence clinical outcomes. These benefits can be maximized if a proper replanning schedule is adopted. Correlation between anatomic deviation and the adverse dosimetric effect during HN radiotherapy has been revealed in many studies [8, 11–13, 23]. ART should therefore be deployed according to the anatomic changes monitored throughout the treatment course. Figure 9a shows the progressive volume loss of the P-NP over time. Increased aeration of the nasopharyngeal region was observed when treatment progressed, and the opening of the Eustachian tube, the torus tubarius and the fossa of Rosenmüller reappeared due to tumor shrinkage. In this study, the P-NP experienced volume loss with a mean loss rate at 0.99%/day and a mean volume loss of 35.70% by the end of the treatment course. Moreover, the initial P-NP volume was negatively correlated with its percentage volume loss. To our knowledge, only one study has been conducted to monitor the gross tumor shrinkage trend in head and neck cases, while none has been performed specifically for NPC cases. In the study by Barker et al. [5] on HN patients, the gross tumor volume (GTV) had a median volume loss of 69.5% and a median loss rate of 1.7%/day. They also showed that the initial GTV was positively correlated with its rate of volume loss, which is contradictory to the result found in the current study. These discrepancies might be due to the heterogeneity of primary treatment sites in the study by Barker et al. This highlights the need to characterize the trends in anatomic changes, particularly in NPC cases, for precise establishment of an ART strategy in this group. MVCT images showed that the anterior portion of the P-NP had noticeable volume regression, i.e. became thinner throughout the treatment course (Fig. 9a). This suggested that the P-NP shrinkage was asymmetric and predominantly along the vertical axis. However, this effect was not great enough to be manifested in the statistical data, and thus the results of the P-NP COM displacement were not used for defining the ART strategy.
Fig. 9.
MVCT slices demonstrating the progressive anatomic changes in P-NP (a), parotid glands (b and c) and neck region (d) over the treatment course. The initial volume is displayed in purple and the updated volume is in yellow for (b), (c) and (d).
Unlike the study of Wang et al. [23], who showed a faster drop in parotid gland volume in the first 3 weeks of radiotherapy, the present study demonstrated a gradual parotid volume loss over time. In addition, their systematic displacements in the medial and superior aspects were also noted throughout the treatment course. The decrease in parotid volume was due to radiation-induced apoptosis that resulted in rapid depletion of the parotid acinar cells [24]. Wang et al. [23] and Vasquez Osorio et al. [8] revealed the relation between planned mean parotid dose and its volume loss during HN cancer treatment, in which parotid glands with a higher planned mean dose suffered a larger volume loss than those with a lower planned mean dose. Similar findings were seen in the present study. At the 9th, 19th and 29th fractions, the accumulated mean parotid dose was estimated as ∼8.0, 17.0 and 26.0 Gy, with a percentage parotid volume loss of 16.70, 30.94 and 43.03%, respectively. Han et al. [13] were so far the only group that had quantified the trend in volumetric changes of parotid glands specifically in NPC patients. They found that the parotid volume reduced at a mean rate of 1.1%/day and had a mean loss of 40.2% by the end of treatment. This is largely consistent with the current study, which showed that the parotid volume decreased by 1.35%/day and 47.54% by the end of the treatment. Han et al. also monitored the actual dose delivered to the parotid glands throughout the treatment course and found a negative correlation between the parotid volume and its median dose. When the parotid gland experienced shrinkage and medial displacement during the treatment course, it consequently translated into the target dose region and received a higher actual dose than expected. This finding gave support for taking parotid volume into consideration during establishment of the optimum replanning strategy in the current study, as substantial change in the parotid volume would signal significant parotid dose variation that required replanning.
The medial displacement of the parotid glands during HN radiotherapy has been well documented [5, 7, 11, 12]. Barker et al. [5] and Lee et al. [7] showed a median medial shift of 0.31 cm and 0.26 cm by the end of the treatment course, respectively, which are close to our findings. Additionally, the present study demonstrated the tendency of parotid shifting to the superior aspect. Progressive MVCT images showed that the parotid shrinkage occurred mostly in the lateral and inferior portions (Fig. 9b and c), which is consistent with the reports from Vasquez Osorio et al. [8] and Robar et al. [11]. Vasquez Osorio et al. [8] divided the parotid glands into six different subvolumes for investigation. They found that although all regions of the parotid tended to move inward when it shrank, the largest displacement occurred in the lateral and inferior regions. The mean displacements were 0.3 cm in the lateral region towards the medial aspect and 0.3 cm in the inferior region towards the superior aspect, whereas the medial region partially adjacent to the bony structures showed least inward translation (0.1 cm). Robar et al. [11] also assessed the geometric changes separately in the medial and lateral aspects of the glands during the radiotherapy course. A systematic medial translation was clearly observed in the lateral aspect of the glands but not in the medial aspect. During the radiotherapy course, the parotid glands could be displaced by the regressed lymph nodes or decreased neck volume [9, 10]. The parotid itself could also shrink asymmetrically, causing its displacement predominantly in these directions, which was also observed in the present study. Follow-up study should be conducted to quantify the subvolume changes.
Figure 9d shows the alterations in the neck habitus throughout the treatment course in MVCT images. The neck volume decreased progressively over time and resulted in a significant drop by treatment end. This increased the risk of patient mispositioning inside the immobilization mask. The lateral cord displacement remained minimal as patients were aligned to the midline marked on the mask. However due to the loss of subcutaneous fat in the neck region, patients frequently shifted backward, which consequently led to posterior displacement of the whole cervical spine as seen in the present study. This effect was also demonstrated by measuring the thickness of the posterior neck at VC3, VC6 and VC9 levels. Figure 10 shows their progressive decrease throughout the treatment course. At treatment completion, the mean posterior neck thickness at VC3, VC6 and VC9 levels dropped by 6.71 ± 7.04%, 9.76 ± 9.42% and 11.00 ± 8.46%, respectively. Consistent with the COM posterior displacement, greater variation was found in the lower neck region (VC9) than the upper neck region (VC3) during the treatment course. Similar results were presented by Robar et al. [11] and Zhang et al. [25], in which both studies also demonstrated largest displacement in the lower neck region. This implies that the geometric displacement of the cervical cord is non-rigid and therefore cannot be completely corrected by couch offset, even with an IGRT approach. A replanning strategy is essential in order to correct for these geometric changes.
Fig. 10.
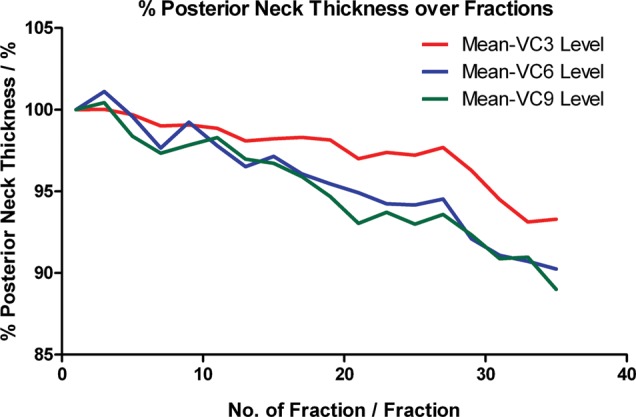
Relative posterior neck thickness over the treatment course.
Capelle et al. [26] pointed out that NPC patients should be targeted for routine adaptive replanning, as the large anatomic changes demonstrated in this group of patients indicates the greatest benefits from plan adaptation among all HN cancers. This study utilized an objective approach to define the ART schedule for NPC cases. Thresholds indicating significant anatomic changes and thus the need for plan modification were spotted out for each selected parameter under statistical analysis. The ultimate ART strategy was established with practical consideration to enhance efficiency and efficacy in the clinical environment. In this study, three replans at the 9th, 19th and 29th fractions are proposed. As in our previous study, multiple replannings during the treatment course were indicated as necessary [17]. Nevertheless, this study indicated one more replan that, if performed at an earlier stage of the treatment course, would allow better rectification of the potential overdose due to early anatomic deformations. Replanning using an appropriate schedule guarantees a prompt reaction to the shrunk tumor by conforming the dose tightly to the targets, thus providing opportunities for safe dose escalation without jeopardizing the surrounding OARs. This should lead to an overall improvement in dosimetric and, in turn, clinical outcomes in NPC cases [27, 28].
ART on an interval basis is feasible in NPC cases because the organ changes, as shown in this study, follow progressive trends. The proposed ART strategy provides further dosimetric improvement on top of that of IGRT, with an acceptable increase in departmental workload and overheads. As the exact fractions for plan modification are already defined, the process of rescanning patients, recontouring structures and replanning need be repeated only three times within the whole treatment course. This is clinically feasible and also greatly reduces the demands on clinical resources, e.g. machine occupancy, and on the oncologists', physicists' and dosimetrists' workloads when compared with a daily adaptive approach. On the other hand, for centers without in-room CT facilities, the implementation of ART would be difficult, because the patient anatomic changes during the treatment course cannot be effectively monitored. The proposed replanning strategy provides a standard guideline for these centers so that ART can be adopted more easily to improve the dosimetric outcome for NPC patients. Although this study was conducted in the tomotherapy unit, the ART strategy can also be applied in NPC cases that are treated with other linear accelerators.
Wu et al. [29] assessed the efficiency of different ART strategies by applying the single mid-course replanning, alternate week replanning (two replans) and weekly replanning (six replans) to 11 patients during HN-IMRT. They concluded that significant dosimetric improvement could be achieved by increasing the replanning frequency from one to two, but not from two to six. Moreover, it was not recommended to conduct replanning more than once a week. This is consistent with the findings in the current study, which also emphasized the necessity for multiple replannings (i.e. >1) and suggested three plan modifications throughout the treatment course. Wu et al. additionally stated that rapid adaptation, i.e. using the new plan within the same week would further improve the dosimetric results. An appropriate replanning schedule in NPC cases is crucial because the timing of initiation of a replan can greatly affect the efficiency of the ART process. If the plan modification is conducted too early, e.g. at the beginning of the course, it is likely that no apparent dosimetric benefits will be seen as the anatomic changes are still minimal; manpower and clinical resources for replanning would be wasted. If the plan modification is conducted at a very late stage in the treatment, irreversible dose changes may result because there would have been no chance to accommodate the anatomical changes that appeared mid-treatment course. This study identified appropriate timing for the initiation of ART procedure during an NPC treatment course. Replannings at these selected time-points should maximize the benefits of ART application in NPC cases.
Several studies have demonstrated a patient-specific way of implementing ART on an interval basis [30–32]. Instead of applying a standard protocol, an individualized replanning schedule was tailor-made for each patient according to his/her own anatomic changes during treatment. The process of replanning was initiated once a significant change was observed. Schwartz et al. [30] report concerning 22 oropharyngeal cancer patients, for whom the replanning process was instigated during dose recalculation from the daily CT images whenever there were significant changes in anatomy which resulted in a geographical miss of the target or inadequate sparing of OARs. In their study, all patients required one replan, and the median trigger point was the 16th fraction. At the time of the first replanning, the bilateral parotid volumes and the CTV had shrunk by 16% and 5%, respectively. Eight patients required two replans with the median trigger points at the 11th and 22nd fractions, respectively. At the time of the second replanning, the bilateral parotid volumes and the CTV had a respective decrease of 24% and 14%. Jensen et al. [31] also implemented an adaptive strategy for HN cancers. Plan modification would be performed if unsatisfactory target volume and OAR position (deviation ≥1 cm) was noted in at least three specific CT slices (base of skull, C3 and supraclavicular fossa) during weekly treatment verification. In their study, 15 out of 72 patients required plan modification during the treatment course, and the number of replans ranged from 1–3. However, the exact time for replanning was not reported. Zhao et al. [32] conducted an ART study on 33 NPC cases. The rescanning and replanning processes were applied to these patients when significant anatomic changes were noticed by inspection, palpation and/or direct endoscopy during the radiotherapy course. The adaptive procedure was triggered after an average of 15 fractions. In particular, nine patients required a third CT scan and a second replan after another 12 fractions. Patient-specific ART provides a customized way to address a patient's anatomic changes, and in turn dose deviation, during the treatment course. Nevertheless, this approach heavily depends on clinical judgment of the ‘significant change’, which can be vary between different centers and even between different oncologists. Also, continuous monitoring of the organ and dose changes during the treatment course is required. From a practical viewpoint, advance technologies like in-room CT machines for fast image acquisition and deformable image registration software for automatic contouring [33–35] are prerequisite for facilitating the process. Moreover, the frequency and timing of plan modification is unpredictable, and therefore allocation of manpower has to be considered for actual implementation. All these factors could tremendously increase departmental burdens. In contrast, the present study demonstrates a simpler approach to ART implementation. By recognizing the trends for different organ changes in advance, the proposed replanning schedule can be applied in all centers for NPC cases, knowing that problematic anatomical changes will be adequately corrected for without daily image guidance.
This study suggested that the initial GTV could be a helpful pretreatment predictor, because large gross tumors tend to shrink more slowly over time. Fang et al. [36] demonstrated that the initial primary tumor volume is negatively correlated to its regression rate, with the correlation coefficient highly consistent with the current study (−0.402 vs − 0.4522, respectively). They suggested that the persistence of a large tumor volume during or after radiotherapy may be due to the fact that dead cell clearance in large tumors after irradiation is less efficient than that in small tumors. This study showed that the initial parotid volume was not significantly correlated with its percentage loss. However, additional work demonstrated its predictive value for absolute volume loss (correlation coefficient = 0.777; P < 0.001). Apparently a larger initial parotid volume leads to a greater absolute loss by treatment completion. These results are in agreement with the findings reported by Broggi et al. [37], who conducted a multivariate analysis to define the possible predictors of parotid shrinkage during HN radiotherapy. They showed the initial parotid volume at treatment end is correlated with its absolute change but not its percentage change. These pretreatment predictors can be employed for the refinement of ART strategy in the future. A less rigorous replanning strategy may be used for tumors exceeding a certain volume, as they would have smaller volume loss and in turn smaller dose variation throughout the treatment course. Similarly, more plan modifications within the treatment course may be needed for parotid glands larger than a certain volume, in order to overcome the frequent dose changes due to rapid shrinkage. These predictors can assist in identifying patients with a high risk of significant anatomic and in turn dosimetric deviation during the treatment course. The proposed adaptive strategy may therefore be refined for specific groups of patients.
Although the image quality of the MVCT is inferior to standard kilovoltage CT (kVCT), the superb contrast between the tissue/air and the tissue/bone allows accurate delineation of the posterolateral wall of nasopharynx and the vertebral canal. These structures were used as the surrogates for monitoring the anatomic changes in the primary gross tumor and the spinal cord, respectively, in this study. Techniques for improving the contrast resolution in MVCT images are rapidly evolving, and precise target delineation in these images will be soon achieved. In this study, the consequent dosimetric outcomes at the proposed replanning fractions are not estimated. In view of the close proximity between target and OARs, the sharp dose gradient at their boundaries in IMRT treatment increases the treatment sensitivity to their anatomic changes. It is therefore believed that significant changes in their size and location at these fractions would cause corresponding important dose deviation such that replans should be proposed. Nevertheless, the dosimetric impact of spinal displacement may be smaller compared with other monitored parameters. Ongoing study will be focused on tracking the volumetric as well as the dosimetric changes of the actual targets and other critical organs during treatment. Updated results will be presented in the near future. In the current study, the mean parotid displacement towards the superior aspect is smaller than the slice thickness for MVCT sets (2 mm vs 6 mm), which may introduce a loss of result reliability. Nonetheless, the shifting trend is clearly visible in the MVCT images, and with reference to the findings of other papers, our result seems reasonable. Further confirmation can be conducted with MVCTs of finer slice thickness.
CONCLUSION
By analyzing the characteristic trends in anatomic changes in NPC cases, this study developed a pragmatic ART strategy specifically for this disease. Three replans at 9th, 19th and 29th treatment fractions were proposed, which could effectively accommodate the anatomical deviation throughout the treatment course. This prevents potential OAR dosimetric consequences and also allows safe target dose escalation. The proposed ART strategy is practically feasible with an acceptable increase in departmental workload and overheads. The proposed strategy is believed to offer a benefit in the current clinical setting, and should be promoted, especially in centers where an adaptive planning system is not yet available.
ACKNOWLEDGEMENTS
This research has been presented in a poster at ESTRO 2012 (9–13 May 2012) and orally at the Hong Kong Sanatorium and Hospital Scientific Meeting in Radiotherapy 2012 (21–22 September 2012).
REFERENCES
- 1.Ma CMC, Paskalev K. In-room CT techniques for image-guided radiation therapy. Med Dosim. 2006;31:30–9. doi: 10.1016/j.meddos.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Oelfke U, Tucking T, Nill S, et al. Linac-integrated kV-cone beam CT: technical features and first applications. Med Dosim. 2006;31:62–70. doi: 10.1016/j.meddos.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Morin O, Gillis A, Chen J, et al. Megavoltage cone beam CT: system description and clinical applications. Med Dosim. 2006;31:51–61. doi: 10.1016/j.meddos.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Schubert LK, Westerly DC, Tome WA, et al. A comprehensive assessment by tumor site of patient setup using daily MVCT imaging from more than 3800 helical tomotherapy treatments. Int J Radiat Oncol Biol Phys. 2009;73:1260–9. doi: 10.1016/j.ijrobp.2008.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker JL, Garden AS, Ang KK, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59:960–70. doi: 10.1016/j.ijrobp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Fang FM, Tsai WL, Go SF, et al. Implications of quantitative tumor and nodal regression rates for nasopharyngeal carcinomas after 45 Gy of radiotherapy. Int J Radiat Oncol Biol Phys. 2001;50:961–9. doi: 10.1016/s0360-3016(01)01531-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee C, Langen KM, Lu W, et al. Evaluation of geometric changes of parotid glands during head and neck cancer radiotherapy using daily MVCT and automatic deformable registration. Radiother Oncol. 2008;89:81–8. doi: 10.1016/j.radonc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Vasquez Osorio EM, Hoogeman MS, Al-Mamgani A, et al. Local anatomic changes in parotid and submandibular glands during radiotherapy for oropharynx cancer and correlation with dose, studied in detail with nonrigid registration. Int J Radiat Oncol Biol Phys. 2008;70:875–82. doi: 10.1016/j.ijrobp.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 9.Kuo YC, Wu TH, Chung TS, et al. Effect of regression of enlarged neck lymph nodes on radiation doses received by parotid glands during intensity-modulated radiotherapy for head and neck cancer. Am J Clin Oncol. 2006;29:600–5. doi: 10.1097/01.coc.0000239093.95769.b3. [DOI] [PubMed] [Google Scholar]
- 10.Kodaira T, Tomita N, Tachibana H, et al. Aichi Cancer Center initial experience of intensity modulated radiation therapy for nasopharyngeal cancer using helical tomotherapy. Int J Radiat Oncol Biol Phys. 2009;73:1129–34. doi: 10.1016/j.ijrobp.2008.06.1936. [DOI] [PubMed] [Google Scholar]
- 11.Robar JL, Day A, Clancey J, et al. Spatial and dosimetric variability of organs at risk in head-and-neck intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1121–30. doi: 10.1016/j.ijrobp.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Lee C, Langen KM, Lu W, et al. Assessment of parotid dose changes during head and neck cancer radiotherapy using daily megavoltage computed tomography and deformable image registration. Int J Radiat Oncol Biol Phys. 2008;71:1563–71. doi: 10.1016/j.ijrobp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Han C, Chen YJ, Liu A, et al. Actual dose variation of parotid glands and spinal cord for nasopharyngeal cancer patients during radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1256–62. doi: 10.1016/j.ijrobp.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 14.Hansen EK, Bucci MK, Quivey JM, et al. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;64:355–62. doi: 10.1016/j.ijrobp.2005.07.957. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Lu J, Xiong X, et al. Anatomic and dosimetric changes during the treatment course of intensity-modulated radiotherapy for locally advanced nasophayngeal carcinoma. Med Dosim. 2010;35:151–7. doi: 10.1016/j.meddos.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Kim K, Park S, Cheong K, et al. Dosimetric changes of intensity modulated radiotherapy (IMRT) plan on the follow-up CT acquired during treatment in the patients with nasopharynx cancer. Eur J Cancer Suppl. 2009;7:487–8. [Google Scholar]
- 17.Fung WWK, Wu VWC, Teo PML. Dosimetric evaluation of a three-phase adaptive radiotherapy for nasopharyngeal carcinoma using helical tomotherapy. Med Dosim. 2012;37:92–7. doi: 10.1016/j.meddos.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Blanco AI, Chao KSC, El Naqa I, et al. Dose–volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1055–69. doi: 10.1016/j.ijrobp.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 19.Eisbruch A, Kim HM, Terrell JE, et al. Xerostomia and its predictors following parotid-sparing irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 20.Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–87. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 21.Chan JKC, Pilch BZ, Kuo TT, et al. Tumors of the nasopharynx: introduction. In: Barnes L, Eveson JW, editors. Pathology and Genetics of Head and Neck Tumours (World Health Organization Classification of Tumours) Lyon, France: IARC press; 2005. pp. 82–4. [Google Scholar]
- 22.Wei WI, Sham JST, Zong YS, et al. The efficacy of fiberoptic endoscopic examination and biopsy in the detection of early nasopharyngeal carcinoma. Cancer. 1991;67:3127–30. doi: 10.1002/1097-0142(19910615)67:12<3127::aid-cncr2820671231>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Wang ZH, Yan C, Zhang ZY, et al. Radiation-induced volume changes in parotid and submandibular glands in patients with head and neck cancer receiving postoperative radiotherapy: a longitudinal study. Laryngoscope. 2009;119:1966–74. doi: 10.1002/lary.20601. [DOI] [PubMed] [Google Scholar]
- 24.Konings AW, Copes RP, Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys. 2005;62:1187–94. doi: 10.1016/j.ijrobp.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Garden AS, Lo J, et al. Multiple regions-of-interest analysis of setup uncertainties for head-and-neck cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64:1559–69. doi: 10.1016/j.ijrobp.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Capelle L, Mackenzie M, Field C, et al. Adaptive radiotherapy using helical tomotherapy for head and neck cancer in definitive and postoperative settings: initial results. Clin Oncol (R Coll Radiol) 2012;24:208–15. doi: 10.1016/j.clon.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Teo PML, Leung SF, Tung SY, et al. Dose–response relationship of nasopharyngeal carcinoma above conventional tumoricidal level: a study by the Hong Kong Nasopharyngeal Carcinoma Study Group (HKNPCSG) Radiother Oncol. 2006;79:27–33. doi: 10.1016/j.radonc.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Teo P, Lee WY, Yu P. The prognostic significance of parapharyngeal tumour involvement in nasopharyngeal carcinoma. Radiother Oncol. 1996;39:209–21. doi: 10.1016/0167-8140(96)01739-2. [DOI] [PubMed] [Google Scholar]
- 29.Wu Q, Chi Y, Chen PY, et al. Adaptive replanning strategies accounting for shrinkage in head and neck IMRT. Int J Radiat Oncol Biol Phys. 2009;75:924–32. doi: 10.1016/j.ijrobp.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83:986–93. doi: 10.1016/j.ijrobp.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen AD, Nill S, Huber PE, et al. A clinical concept for interfractional adaptive radiation therapy in the treatment of head and neck cancer. Int J Radiat Oncol Biol Phys. 2012;82:590–6. doi: 10.1016/j.ijrobp.2010.10.072. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L, Wan Q, Zhou Y, et al. The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 2011;98:23–7. doi: 10.1016/j.radonc.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Castadot P, Lee JA, Parraga A, et al. Comparison of 12 deformable registration strategies in adaptive radiation therapy for the treatment of head and neck tumors. Radiat Oncol. 2008;89:1–12. doi: 10.1016/j.radonc.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji SY, Hwang A, Weinberg V, et al. Dosimetric evaluation of automatic segmentation for adaptive IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;77:707–14. doi: 10.1016/j.ijrobp.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Zhang T, Chi Y, Meldolesi E, et al. Automatic delineation of on-line head-and-neck computed tomography images: toward on-line adaptive radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:522–30. doi: 10.1016/j.ijrobp.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 36.Fang FM, Tsai WL, Go SF, et al. Implications of quantitative tumor and nodal regression rates for nasopharyngeal carcinomas after 45 Gy of radiotherapy. Int J Radiat Oncol Biol Phys. 2001;50:961–9. doi: 10.1016/s0360-3016(01)01531-0. [DOI] [PubMed] [Google Scholar]
- 37.Broggi S, Fiorino C, Dell'Oca I, et al. A two-variable linear model of parotid shrinkage during IMRT for head and neck cancer. Radiother Oncol. 2010;94:206–12. doi: 10.1016/j.radonc.2009.12.014. [DOI] [PubMed] [Google Scholar]