Table 3.
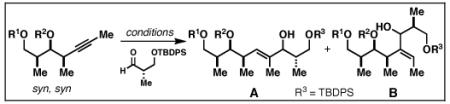
| entry | internal alkyne |
conditionsa | yield | regioselection A:B |
|---|---|---|---|---|
| 1 | 77; R1 = H | A | 65 b | 8:1 |
| R2 = PMB | ||||
| 2 | 78; R1 = TBDPS | A | 68 b | 4:1 |
| R2 = H | ||||
| 3 | 79; R1 = TBDPS | B | 42 | 1.3:1 |
| R2 = PMB | ||||
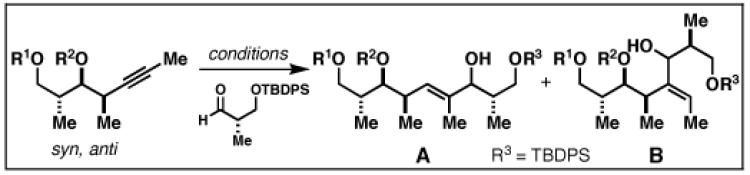
| ||||
| 4 | 80; R1 = H | A | 78 b | 18:1 |
| R2 = Bn | ||||
| 5 | 81; R1 = Bn | A | 58 b | 10:1 |
| R2 = H | ||||
| 6 | 82; R1 = Bn | B | 59b | 4:1 |
| R2 = Me | ||||

| ||||
| 7 | 83; R1 = H | A | 68 | 16:1 |
| R2 = Bn | ||||
| 8 | 84; R1 = Bn | A | 68 | 10:1 |
| R2 = H | ||||
| 9 | 85; R1 = Bn | B | 64 | 4:1 |
| R2 = Me | ||||
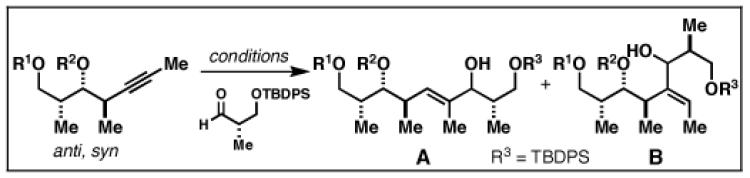
| ||||
| 10 | 86; R1 = H | A | 69 | 20:1 |
| 11 | 87; R1 = TBS | A | 61 | 4:1 |
| R2 = H | ||||
| 12 | 88; R1 = TBS | B | 61 | 2:1 |
| R2 = PMB | ||||
Reaction Conditions: A) nBuLi, PhMe, −78 °C; then ClTi(OiPr)3, cC5H9MgCl, −78 to −40 °C; then BF3•OEt2, −78 °C and aldehyde; B) ClTi(Oi-Pr)3, cC5H9MgCl, −78 to −40 °C; then BF3•OEt2, −78 °C and aldehyde.
Yield reported is for isomer A.
