Fig. 1.
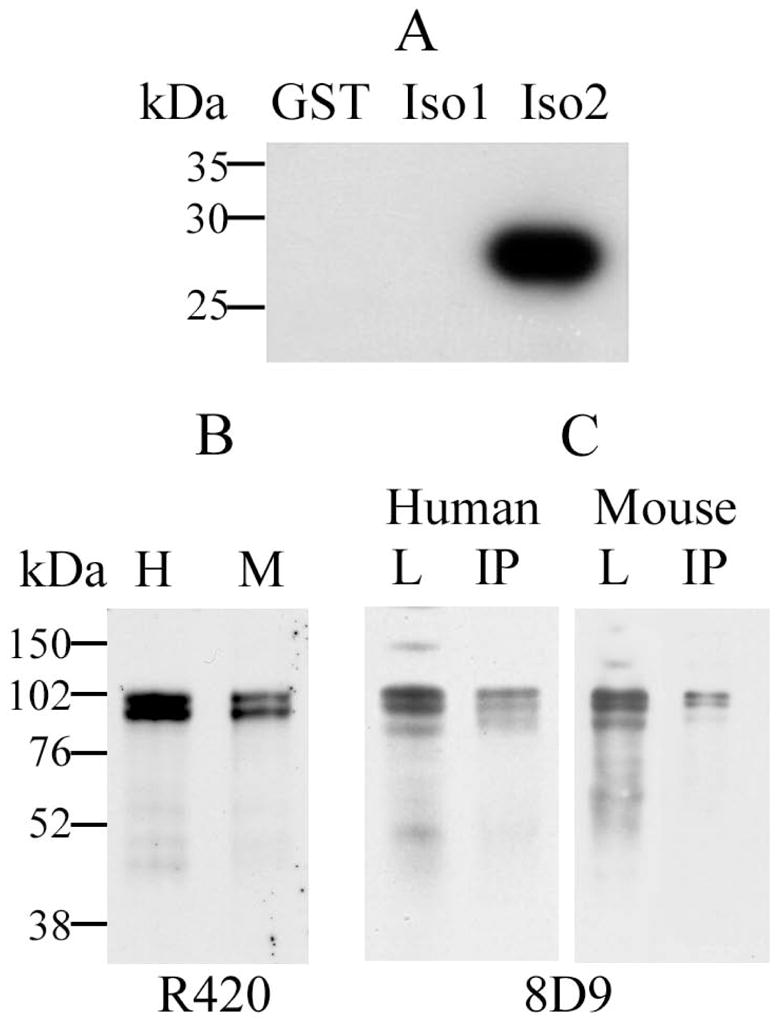
Evaluation of the specificity of affinity-purified pAb R420 by immunoblotting and immunoprecipitation. A: Immunodetection of a recombinant GST (lane GST), and GST fusion proteins containing short fragments of DYRK1A 763-aa isoform 1 (lane Iso1) and 754-aa isoform 2 (lane Iso2) using R420. Twenty ng of antigen was loaded in each lane of 10% SDS-polyacrylamide gel. Specificity of R420 for the DYRK1A isoform 2 was demonstrated by absence of cross-reactivity with the DYRK1A isoform 1. B: DYRK1A protein was detected by R420 as 94–97 kDa bands in lysates from human (H) and mouse (M) brains. C: Specificity of DYRK1A recognition by R420 was confirmed by immunoprecipitation from human brain lysates (Human), and Dyrk1A from mouse brain lysates (Mouse) with R420 (IP); the lysate loads for IP are shown in lanes L. DYRK1A in lanes IP and L was detected with mAb 8D9.
