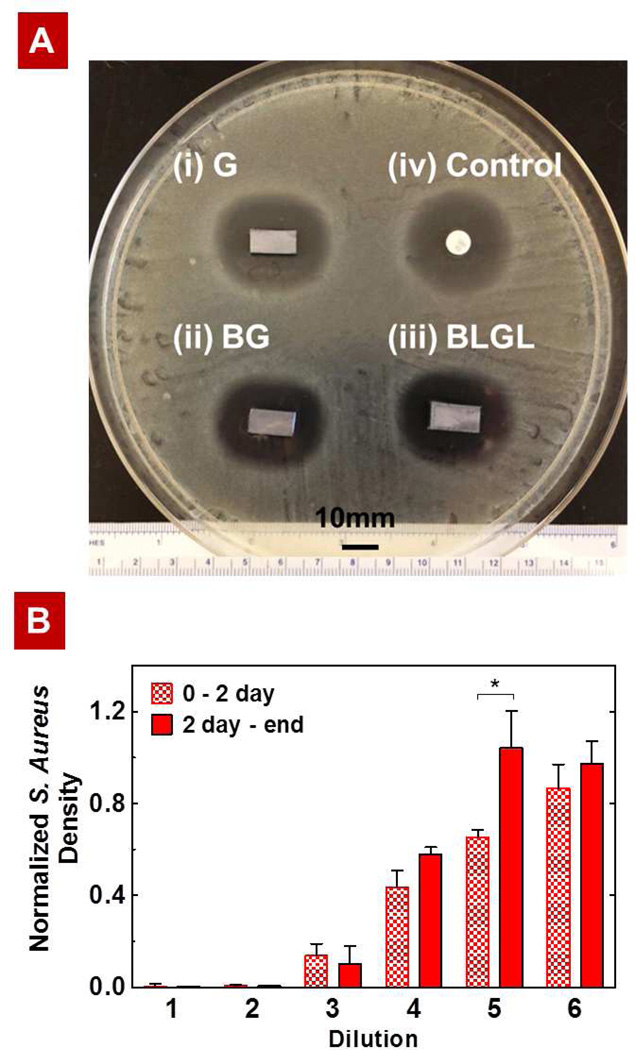
Figure 6.
(A) Comparison of the antibacterial activity of the LbL films with a commercially available BD Sensi-Disc via Kirby-Bauer assay. The silicon substrates coated with (i) G40, (ii) B80G40, or (iii) B80L15G40L15 produced similar zones of inhibition (ZOI) of 25.6 mm against S.aureus (the ZOI is measured perpendicular to the long axis of the substrate). The Sensi-Disc standard with 10 µg of gentamicin, which produces a ZOI of 26.0 mm, served as control. (B) Normalized S. Aureus density upon exposure to dilutions of film release solutions (i.e., release from 0–2 days and from 2 days – end) from the barrier composite film B80L15G40L15 (dilution 1 = 1.0 ± 0.2 µg/mL). Each subsequent dilution is half the concentration of the previous dilution. *P < 0.05, analysis of variance (ANOVA) with a Tukey post hoc test.