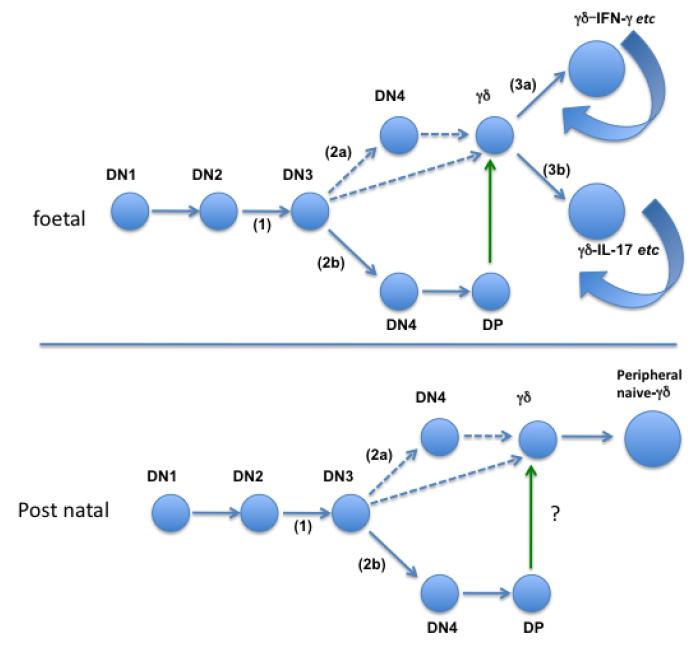
Figure 1. Overview of pre- and post-natal γδ T cell development.
Top: Mouse γδ T cell development from foetal liver progenitors. Cells undergo development through several steps of differentiation, starting at the double negative 1 (DN1) stage characterized by a CD44+ CD25− phenotype, followed by the CD44+CD25+ DN2 stage. At this point, the β, γ and δ chains of the TCR are rearranged. A functional γδ TCR expression will drive cells into the γδ lineage, supported by the expression of Sox13. Cells failing to produce a functional γδ TCR will undergo β selection supported by Notch 1, with a further rearrangement of the TCR α chain, eventually entering the Double Positive (DP) stage. These cells can support γδ T cell development via trans-conditioning (green arrow). Unlike αβ T cells, γδ T cells are functionally pre-programmed, depending on TCR and/or related signalling. It is proposed that a strong, agonist-dependent signal will result in the loss of Sox13 and expression of NFAT, NFκB, Egr3 and Tbet, and a capability to produce IFN-γ among other effector molecules while weaker TCR signalling permits the cells to maintain Sox13, increase Rorγc expression, and a adopt an IL-17 “default position”. The dashed line indicates the potential origin of γδ cells from progenitors with either a DN3 or DN4 phenotype. The curved arrows indicate the potential for lifelong self-renewal that exists in at least two pre-natally derived γδ cell compartments.
Bottom: Mouse post-natal development from bone marrow-derived progenitors. There is no evidence that innate-like CD27− IL-17-producing γδ T cells can be generated from the bone marrow, implying that a different thymic progenitor gives rise to post-natal γδ T cells, by comparison to foetus-derived γδ cells. With no evidence for IL-17-competent progenitors, the evidence for pre-programming is less, and naïve unprimed cells may emerge in the periphery, possibly with a “default potential” for IFN-γ production.