Abstract
Follicle wall rupture and ovum release, i.e., ovulation, has been described as a controlled inflammatory event. The process involves tissue remodeling achieved through leukocyte-mediated proteolysis. In birds, ovulation is the first step in the energy-intensive process of egg formation, yet hens that consume energy in excess of productive requirements experience impaired egg-laying ability. Broiler chickens, selected for rapid lean muscle gain, and coincidentally hyperphagia, develop adult obesity when given free access to feed. Obese broiler hens experience elevated circulating concentrations of insulin and leptin, changes in lipid and lipoprotein metabolism similar to those of human metabolic syndrome, as well as increased systemic inflammation. Overall, the manifestations in poultry are similar to those of women with polycystic ovary syndrome. It was shown recently that, in hens, as in mammals, changes in lipid synthesis and metabolism cause granulosa cell apoptosis and altered immune function and hormone production, further compromising ovarian function. To date, there is insufficient information on the means used by the ovary to direct leukocyte function toward successful ovulation. More information is needed regarding the control of proteolytic actions by leukocytes with regards to the roles of specific enzymes in both ovulation and atresia. The broiler hen has provided unique insight into the interrelations of energy intake, obesity, leukocyte function, and reproduction. Additional work with this model can serve the dual purposes of improving avian reproduction and providing novel insights into polycystic ovary syndrome in women.
Introduction
Birds comprise the highly diverse Aves class within the Chordata phylum. Containing ~27 orders, this review is most correct for birds belonging to the order Galliform of the genus and species Gallus domesticus, namely, the domestic chicken. However, all birds are oviparous and so must prepackage all nutrients needed by the incubating embryo growing within a hard-shelled egg. Disordered fabrication of the egg–embryo biologic unit will compromise reproduction. The egg itself is a complex structure and a marvel of biologic engineering because its proper formation provides both the physical environment and nutrition needed by the embryo to complete incubation successfully. Properly formed, an egg is said to be “settable,” a term that refers to its physical suitability for placement in an incubator. Settable eggs have a single yolk of appropriate size, albumen, intact membranes, and a continuous smooth shell that is neither too thin nor too thick (1). Yolk lipid is synthesized in liver as both VLDLs and very HDLs, whereas albumen, membranes, and shell are synthesized by the oviduct. Ovulation of the ovum (i.e., the yolk) and its presence in the oviduct provides a mechanical stimulus for subsequent egg formation. In this regard, birds differ from leatherback turtles that routinely lay shelled albumen gobs (2) along with yolk-containing eggs.
Egg formation is clearly the outcome of highly integrated multi-tissue processes; it is also a closely timed and energy-intensive process. Despite its high energy requirements, there is little biologic tolerance for excess, and hens in significant acute or chronic positive energy balance quickly reduce the number of settable eggs laid (3–6). These studies showed that excess energy intake in association with fat mass gain disturb the estrogen-induced reprogramming of whole-body lipid and lipoprotein metabolism that support yolk formation. However, these disturbances were only part of the answer to reproductive failure. Overfed hens may exhibit expanded ovarian hierarchies, hyperovulation, increased fractional yolk mass, delayed ovulation, as well as apoptosis and necrosis within ovarian follicles. All of these events underscore that ovulatory processes are disordered beyond a defect in yolk deposition per se. Interestingly, and perhaps because of the need to temporally coordinate ovulation of a single ovum (yolk) with shell formation and oviposition, reduced egg production can occur despite the presence of more hierarchical follicles, in conjunction with laying of double-yolked eggs, partially shelled or shell-less eggs, although ultimately a higher incidence of follicular atresia leads to ovarian involution (3, 7–9)
Obesity leads to increased inflammation, a situation that has been shown to disrupt normal intercellular and intracellular signaling in several chronic diseases, including diabetes and atherosclerosis (10, 11). Disruption in the timing of the individual steps required to fabricate an egg is frequent malfunction and alterations in the timing of ovulation particularly disruptive to the correct sequencing of egg formation. Interestingly, genetic selection of chickens for commercial meat or egg production has resulted in strains of chickens that differ greatly in spontaneous energy consumption and total egg production. To date, ovarian morphology and hormone production, circulating hormonal patterns, as well as hypothalamic–pituitary–ovarian axis responses to hormonal and fuel signals have been compared in hens made lean or obese through genetic selection or dietary manipulations (8, 12–15). However, a fundamental mechanism responsible for poor reproductive efficacy in broiler hens remains elusive. Nevertheless, investigations into how these fundamental differences develop are revealing connections among liver, ovary, and adipose, as well as the white blood cells that circulate among them. This review aims to highlight what is known and the gaps that remain regarding our understanding of how obesity and the systemic inflammation that it causes disrupt the tissue–tissue interactions that result in successful egg laying.
Yolk Formation
The egg yolk is the single large haploid cell that is ovulated from the ovarian follicle. Its presence in the oviduct provides a mechanical stimulus for subsequent egg formation. Within the chicken ovary, there is a hierarchy of follicles of increasingly large size and maturity; indeed, the 2 features are linked conceptually in the literature noting whether the ovum has reached “an ovulable size” (9). In connection with size, a primary requirement for the incubating embryo is sufficient energy to grow and develop into a healthy chick. The average chicken embryo requires 64 kcal to form tissues (36 kcal) while generating heat (24 kcal) associated with normal metabolism (16). TG is the most concentrated form of energy, and this is the form used by the incubating embryo. A 50-g egg (USDA large) contains ∼72 and 55 kcal in yolk and 17 kcal provided by the albumen (17). The energy present in yolk comprises ∼22% protein and 74% fat and is deposited via receptor-mediated uptake of TG-rich VLDL (TRL)7 and the phospholipid-rich very HDL vitellogenin (VTG). In chickens, VTG supplies ∼7% of yolk total lipid and 23% of yolk solids, whereas the TRL provides 93% of yolk lipid and 66% of yolk solids (18).
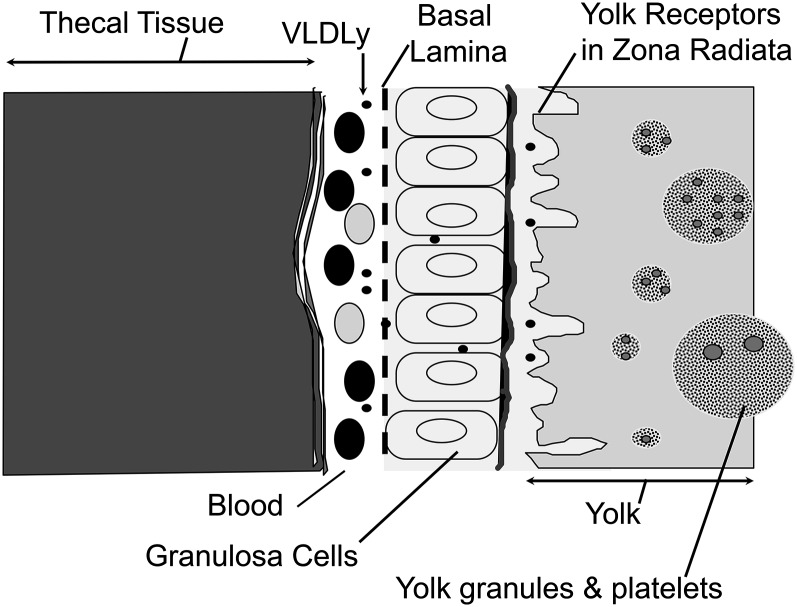
To ensure adequate energy delivery to the yolk follicle, the hen’s liver, under the influence of estrogen, assembles and secretes a highly specialized TRL, called yolk-targeted VLDL (VLDLy), to signify its specific role in targeting lipid nutrients for deposition into yolk (18–20). The VLDLy particle is rich in TG and contains apolipoprotein B100 and apolipoprotein-II of VLDL, the latter of which is a small apoprotein that is made in response to increases in estrogen concentration (21, 22). Apolipoprotein-II of VLDL is thought to control VLDLy diameter and is known to inhibit lipoprotein lipase (LPL) (23, 24), likely by changing the surface of the particle to exclude water needed for lipolysis (25). Lipase resistance ensures delivery of energy-rich TG to the embryo, as does reduction in particle diameter from ∼70 nm of VLDL to ∼27 nm of VLDLy, a change that facilitates passage of the intact lipoprotein particle through the granulosa layer of the follicle and allows it to come into close proximity of the oocyte surface (Fig. 1) (14, 15, 20). VTG is also sufficiently small to pass through this tissue layer and access yolk receptors (26). In laying hens, VLDLy is deposited into the growing yolk follicle by receptor-mediated endocytosis using a type of VLDL receptor that is a member of the LDL receptor (LDLR) superfamily (15, 27). Yolk deposition occurs using a shorter slice variant of the VLDL receptor, termed LR8, which is present on the oocyte surface and is able to bind to both VLDLy and VTG (28). The ability of the coupled VLDLy/LR8 ligand/receptor system to target lipid nutrients for yolk deposition was clearly shown in early work by Bacon et al. (29) in which only 25% of radiolabeled VLDLy isolated from laying hens and injected into sexually immature females was recovered in carcass, whereas 94% of radiolabeled VLDL isolated from sexually immature females and similarly injected was recovered. The essentiality and selectivity of the LR8 receptor/VLDLy receptor/ligand pair for yolk deposition is clearly shown by massive accumulations of VLDLy in the bloodstream of restricted ovulator hens whose LR8 carries a point mutation in the coding sequence of the gene for this receptor (30, 31). These hens rarely form yolks or settable eggs, and, as a result, the mutation is carried through the male line (32).
FIGURE 1.
Diagram of avian yolk follicle in cross-section. Note that ovarian tissue layers (theca and granulosa) are vascularized, providing the means for delivery of hepatic lipoproteins to the oocyte surface (zona radiata) in which lipoprotein receptors are located. Yolk forms by receptor-mediated uptake of intact VLDL and vitellogenin. Only yolk-targeted VLDL, 25–30 nm in diameter and resistant to hydrolysis by lipoprotein lipase, passes through the granulosa basal lamina; vitellogenin is a large phospholipidated protein ∼10 nm in diameter. VLDLy, yolk-targeted VLDL.
Another lipoprotein/receptor pair of critical importance to egg laying is the LDL/LDLR. The avian LDLR ortholog was not characterized until 2003 (33), in part because of the multiplicity of the LDLR superfamily members in ovarian tissue. The ovarian LDLR prefers cholesteryl ester-rich LDL to VLDL and is present in both granulosa and thecal cells in which it is proposed to ensure lipoprotein-derived cholesterol delivery for steroid hormone synthesis. This receptor is also highly expressed in the adrenal glands (34). We could not find publications regarding the effects of energy excess on LR8 or LDLR gene expression or binding activities.
Ovulation
There are several signaling processes under active investigation that may mediate interactions between the ovum and somatic cells such as those found in the granulosa and thecal layers that are involved in follicle selection and maturation; the reader is directed to current reviews by others for more information on this aspect of folliculogenesis (35). As noted in that review, signaling factors and receptors of interest must often be studied using non-homologous reagents, and this technical challenge often limits research progress. This review focuses on ovulation per se, which has been characterized as a controlled inflammatory event (36). The expulsion of the oocyte from the follicle is the outcome of successful ovulation and signals the last step in folliculogenesis. In mammals, it is well documented that leukocytes bound within the ovary act as in situ modulators of ovulation through secretion of regulatory factors, proteases, and phagocytosis (37–39). Ovulation requires intensive tissue remodeling that is primarily dependent on fibroblast mobilization, production of soluble mediators, disintegration of the connective tissue elements, and an acute inflammatory reaction, including leukocyte recruitment, migration, and activation for release of regulatory factors such as cytokines, proteases, and reactive oxygen intermediates (37–39). There is scattered evidence on the role of leukocytes in avian ovulation per se. Nili and Kelly (40) identified macrophages as operative in the process of bursting atresia in which follicle wall rupture is a characteristic (41). Sundaresan et al. (42) investigated the actions of leukocytes in the avian postovulatory follicle, noting the involvement of cytokines and chemokines. In birds, the hierarchical position of ovarian follicles within the most mature or “yellow” hierarchy is indicated by a number, such as F1–F3, in which higher numbers indicate less mature follicles. Barua et al. (43–45) published a series of immunohistologic studies documenting an increase in macrophage numbers within the ovary of young compared with old hens, laying compared with immature hens, and in F1–F3 follicles compared with small white follicles. In each of these comparisons, increases in macrophage number occurred in the more active ovulatory condition. Postovulatory and atretic follicles had the greatest number of macrophages (43, 45). Onagbesan et al. (46) reported that conditioned media collected from LPS stimulated HD-11 avian macrophage cell line cultures mimicked the effects of TNF-α and its interactions with insulin-like growth factor-1 and luteinizing hormone on progesterone production and cell proliferation in cultured granulosa cells. Recently, Cornax et al.(47) showed that primary chicken macrophages quickly phagocytized yolk after in vitro exposure without increases in inflammatory cytokines, such as IL-1, IL-6, and IFN-γ. These same studies showed that yolk injection into the abdominal cavity of young male chickens failed to invoke an inflammatory response. In fact, yolk dampened many inflammatory changes caused by LPS (47). Thus, it seems unlikely that exposure of the follicle wall to yolk precursor VLDLy or VTG during the 7–10 d needed to complete yolk follicle growth provokes a generalized inflammation that eventually ruptures the follicle wall when the yolk is sufficiently large. Indeed, some form of alternative activation may occur in macrophages involved in ovulation to achieve tissue-specific outcomes (48). In mammals, several secretory and membrane-type matrix metalloproteinase (MMP) and their inhibitors (such as tissue inhibitor of MMP), have been shown to increase in expression and activity in conjunction with follicle development (49). Because of tight coordination in matrix degradation before ovulation and the redundant actions of various MMPs with overlapping substrate specificity, few have been demonstrated as obligatory for follicle wall remodeling before ovulation in mammals. Through an in vitro ovulation system, gelatinase A and membrane-type MMPs were shown to be responsible for follicle rupture during ovulation in the medaka. In that species, gelatinase A was shown to degrade type IV collagen in the follicular basement membrane, membrane-type 2 MMP was shown to hydrolyze theca layer type I collagen, and MT1–MMP and tissue inhibitor of MMP-2 were responsible for gelatinase A production and activation in medaka fishes (50). Similar information is needed for avians.
Two Types of Hens
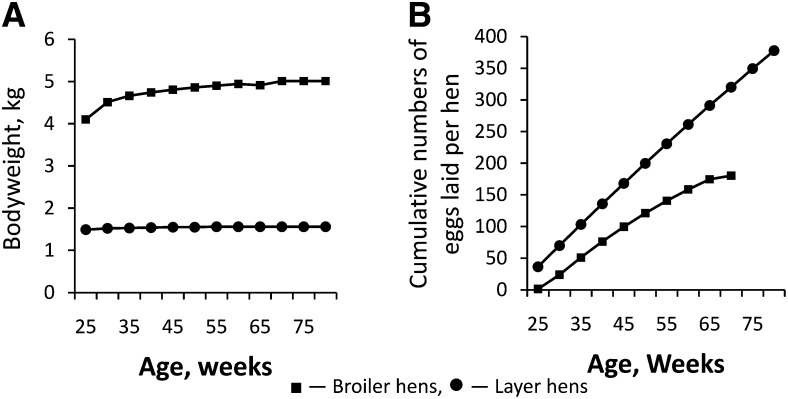
Hens that have been genetically selected for egg production (so called “table-egg” or “layer” strains) have small body size (1500 g), tight control of feed intake, and lay ~364 eggs during a 60-wk production cycle, or 6 eggs/wk (Fig. 2A) (51). In marked contrast, hens from strains selected for meat production (so called “meat-type” or “broiler” strains) are heavy bodied (4000 g), tend to overeat, and, with proper management, can lay up to 180 eggs during a 44-wk production cycle, or 4 eggs/wk (Fig. 2B) (52). Proper nutritional management of broiler breeder hens includes feed restriction to limit energy intake. The propensity of broiler hens to lay fewer eggs at irregular intervals with more shell abnormalities was well known by 1968 (53). Jaap and Muir (53) documented this propensity for several growth-selected (broiler) lines compared with layer strains. The title of their paper “Erratic Ovulation and Egg Defects in Broiler-Type Pullets,” gave rise to the currently used erratic ovulation and defective egg syndrome (54) describing this tendency. Although Jaap and Muir noted that broiler pullets were larger than layers and that genetic selection had caused progressive divergence in the body sizes in chickens purposed differently in food production systems, no mention of body fatness was made, but the authors commented that feed restriction was not used in their studies as was common practice in commercial broiler breeder operations of the time. A series of publications by Yu and Robinson and others (6, 13, 55) provide the most comprehensive documentation of the profound declines in egg production and associated changes in ovarian morphology and hormone production when broiler hens are allowed free access to feed. Twenty years later, equivalent observations are made with current breeder strains allowed to chronically overeat (3, 4). Broiler hens reared under feed restriction and subsequently provided with free access to feed increase feed intake from 145 to 290 g/d for 2 d, slowing to a steady intake of 250 g/d (3).
FIGURE 2.
Innate differences in bodyweight (A) and egg production (B) in well-managed broiler and layer hens. Broiler hen management for egg production requires control of bodyweight by feed restriction starting at 3 wk of age because this type of hen spontaneously consumes excessive amounts of feed and becomes obese as an adult. Layer hen management provides for ad libitum feed consumption because this type of hen self-regulates feed intake for optimal egg production. Mature lean broiler hens are ~2.5 times as large as layer hens as indicated by bodyweight at 25 wk (A). Layer hens lay more eggs during any given time interval and persist in egg laying longer than broiler hens (B). Data are adapted from information found in references 51 and 52.
Laying strains were likewise known to develop fatty liver syndrome, at times so severe that fatal hemorrhage also occurred (also known as fatty liver hemorrhagic syndrome) (56–58). The syndrome most often occurred transiently when highly productive flocks experienced sudden periods of hot weather and resolved when hens’ reduced feed intake or feed energy concentration was reduced (19). It was proposed that reduced energy requirements of hens during hot weather caused typical energy intake to be excessive and provocative of fat accumulation in the liver. During these episodes, settable egg production would drop precipitously. Similar changes in liver fat and egg production can be provoked in egg-type hens if they are intubated with 50% more feed than spontaneously consumed (5, 58). Thus, whether overfeeding was implemented by environmental misadventure, intubation, or allowing spontaneous overconsumption, overfed layer and broiler hens manifest similar changes in ovarian functions and lipoprotein profiles. Energy excess in the form of accumulated adipose was also associated with reduced egg production at the end of commercial egg production cycles. In some operations, a second period of high egg production is brought on by molting in which hens are caused to lose ∼30% of bodyweight to increase subsequent egg production (59, 60). Notably, this practice is based on avian physiology; feed intake is spontaneously reduced in natural molt (61).
Obesity
The term obesity was not in routine use in the poultry literature until 1993 (58). Indeed, there are no universal criteria for obesity in domestic fowl, nor was there an association between body fatness in hens and increased inflammation until 2006 (3). However, before this, it was widely recognized that excess energy intake caused changes in FA and acylglycerol metabolism. In chickens, the liver produces >80% of adipose tissue FAs (62) and is the only organ that responds to feeding/fasting manipulations of insulin signaling (63); the diet of chickens is typically very low in added fat, i.e., often <6% of total calories. For these reasons, alterations in tissue and lipoprotein lipid composition is primarily reflective of changes in endogenous FA synthesis and metabolism. Detailed examination of lipoprotein profiles in overfed layer hens showed increased peripheral lipolysis of hepatic TRL and loss of yolk targeting of VLDLy (5). Particle diameter of VLDLy increased from ~30 to ~40 nm, the amount of the TRL lipolytic end product LDL increased, as did amounts of circulating HDL. In highly productive hens, the amount of HDL decreases because, despite high TRL concentrations, TRL in the form of VLDLy is poorly lipolyzed and produces little LDL, a process that generates excess surface phospholipid for transfer to HDL via phospholipid transfer protein and so promotes HDL maturation (19, 64). Importantly, despite the increase in circulating HDL concentration, the LDL from overfed hens was shown to be smaller and denser, as was the HDL when compared with those from control hens. These changes were reminiscent of those associated with metabolic Syndrome X in humans. This impression was confirmed in subsequent studies with broiler hens provided with free access to feed for 10 d, which became obese and developed fatty liver (similar to human non-alcoholic fatty liver), hyperglycemia, hyperlipidemia, hyperinsulinemia, hyperleptinemia, and elevated non-esterified FAs (NEFAs). Insulin resistance occurred in conjunction with poor egg production, high mortality, and abnormal ovarian morphology (3). Leptin is a proinflammatory hormone with a number of systemic effects that are distinct from suppression of food intake (65, 66). In mammals, obesity-associated metabolic changes similar to those noted above have been termed lipotoxicity and are thought to underlie pancreatic β-cell failure and progression from insulin resistance to overt diabetes (67). Targeted metabolomics of acylglycerols in tissues, NEFA, and lipoproteins in broiler hens showed that ovarian dysfunction was associated with increased proportions of SFAs in liver, NEFA, and VLDLy (3). Susceptibility to poor egg production and abnormal ovarian morphology in broiler hens provided with free access to feed increased with decreasing stearoyl-CoA desaturase activity as indicated by reduced 16:1n7/16:0 or 18:1n9/18:0 in NEFA (3). These findings supported the notion that changes in the molecular species of FAs produced by the liver play a role in avian ovarian follicle dysfunction; similar FA effects has been observed in humans (68). Granulosa cells isolated from broiler hens in that study consuming unlimited rather than restricted amounts of feed showed striking increases in apoptosis and necrosis during a 12-h in vitro incubation (3).
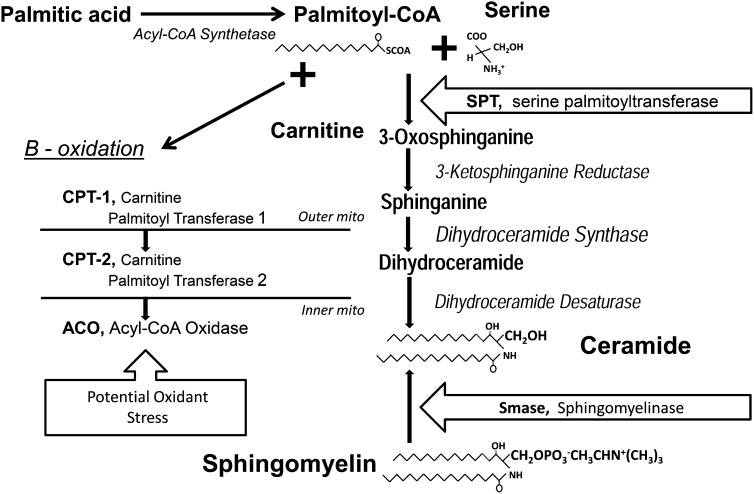
Broiler hens that were brought into egg production using breeder-recommended feed restriction until 27 wk of age and then allowed free access to feed for 3 wk showed elevated testosterone concentrations in conjunction with reduced estrogen and progesterone (69). Although leptin concentrations were not measured in that study, hyperleptinemia has been demonstrated to occur in similarly treated broiler hens (3). Leptin negatively affected rodent granulosa cell steroidogenesis and follicle maturation in vitro (70, 71), whereas rats treated with leptin exhibited significantly impaired in vivo and in vitro ovulation (72) Subsequent studies with broiler hens reared using feed restriction to 26 wk of age and then provided free access to feed for 9 wk and then an additional 24 wk accumulated ceramide and sphingomyelin in liver and circulating TRL and exhibited increased proinflammatory interleukin-1β gene IL1β expression in liver and adipose tissues (4). Furthermore, IL1β, serine palmitoyltransferase, and sphingomyelinase transcript levels were increased, but serine/threonine protein kinase activation was decreased within the hierarchical follicles. These studies were the first to suggest that the aberrant metabolic programs limiting egg production in broiler breeder hens were similar to those in obese, insulin-resistant, or overtly diabetic mammals, including women with polycystic ovary syndrome (68) whose metabolite and endocrine profiles include insulin resistance, hypertriglyceridemia, increased circulating concentrations of ceramide and NEFA with increased proportions of SFAs, and systemic inflammogens, including elevated hepatic, adipose, and ovarian IL-1β production. In vitro studies showed that physiologically high concentrations of palmitic acid were overtly toxic to granulosa cells (73). In these studies, palmitate-induced cell death was accompanied by increased acyl-CoA oxidase, carnitine palmitoyl transferase-1, serine palmitoyl transferase, and sphingomyelinase gene transcription in conjunction with increased concentrations of the proinflammatory IL-1β. Triacsin-C inhibition of fatty acyl-CoA synthesis completely rescued granulosa cultures from palmitate-induced cell death. Partial to complete prevention of cell death was also seen with addition of the free radical scavenger pyrrolidine dithiocarbamate, the sphingomyelinase inhibitor imipramine, or the de novo ceramide synthesis inhibitor fumonisin B1, supporting the notion that palmitate-induced granulosa cell cytotoxicity operates through a palmitate-derived metabolite. Palmitoyl-CoA may be channeled into β-oxidation and/or into formation of bioactive metabolites that increase free radical generation, inflammatory response, and ceramide production. Thus, palmitate-derived metabolites appear to activate apoptotic machinery in granulosa cells, whose death results in follicular atresia and reduced egg production in fuel-overloaded broiler breeder hens. It seems that both increased synthesis and sphingomyelin remodeling contribute to tissue ceramide accumulation (Fig. 3). Local and systemic inflammation and immune responses (74) were shown to be mediated by IL-1β in response to leptin in many cell types, including granulosa cells (75). IL-1β stimulates sphingomyelin hydrolysis and de novo ceramide synthesis, leading to cell apoptosis. In cultured human granulosa cells, IL-1β inhibited estradiol production and accelerated pregn-4-ene-3,20-dione degradation (76, 77). Palmitoleate was shown to reduce liver inflammatory response in mice (78), consistent with the observation that hens with elevated hepatic stearoyl-CoA desaturase activities are resistant to the deleterious effects of excess energy consumption on egg production (3).
FIGURE 3.
Biochemical pathways involved in creation of the bioactive lipids that negatively affect reproductive function. Overconsumption of high-carbohydrate feed stimulates lipogenesis and palmitic acid formation. Palmitate can either be catabolized through β-oxidation and so potentially increase oxidant stress in the mitochondria or combined with serine to form ceramide through the actions of serine palmitoyltransferase. Sphingomyelin can be remodeled to ceramide by sphingomeylinase. The obesity-associated inflammatory cytokine interleukin-1β gene stimulates ceramide formation. Palmitate and ceramide cause inflammation, prompt apoptosis, and reduce hormone production through granulosa cell death.
The cytotoxicity induced by excessive SFAs that operate in broiler breeder hens appears similar to those in mammals (79–81). Interestingly, oleic and linoleic acids also induced granulosa cell apoptosis but to lesser degrees depending on the degree of unsaturation of the 2 FAs (80). This last report underscores the critical role of actual FA composition and concentration of plasma NEFA and VLDL lipids in SFA-induced cell apoptosis in vivo. In this regard, it is important to recall that the laying hen granulosa cell LPL activity can be as much as half that of adipose LPL and >10-fold higher than the adjacent theca cells (82). Notably, VLDLy resists lipolysis but is not impervious to this process, nor are all VLDL in overfed hens lipase-resistant VLDLy, as indicated by increased particle diameter. These functional and anatomical relations highlight the critical role of cellular FA disposal with the multi-tissue structure that comprises the avian ovary and in follicular atresia within the yolk hierarchy.
Conclusions
Obesity in hens appears to provoke reduction in settable egg formation by setting a metabolic chain reaction in motion that propagates from the liver through delivery of bioactive FAs and other bioactive lipids to peripheral circulation and ultimately peripheral tissues. Bioactive lipids, including palmitic acid, sphingomyelin, and ceramide, provoke inflammation and alter cell signaling processes in ways that lead to granulosa cell dysfunction and death, altered timing of ovulation that subsequently impairs egg formation by disruption of the steps leading to settable egg formation. Susceptibility to ovarian disruption during obesity is individual in both layer and broiler hens and dependent on lipid metabolism. Many of the changes observed to date are similar to those observed in mammals, particularly women with polycystic ovary syndrome. Although it seems clear that leukocytes are involved in avian ovarian function, there is only a rudimentary understanding what functions they perform and how they affect avians’ ovulatory processes, which in the case of highly productive layer hens is a near daily event. The multiplicity of tissues and processes involved in forming a settable egg is impressive and only partially resolved. A better understanding of the critical regulatory steps and interactions may allow improved selection strategies to raise broiler hen productivity or lead to new management strategies to improve production in commercial settings, as well as potentially improve conservation efforts in wild species.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: LDLR, LDL receptor; LPL, lipoprotein lipase; LR8, short splice variant of LDL receptor; MMP, matrix metalloproteinase; NEFA, non-esterified FA; TRL, TG-rich VLDL; VLDLy, yolk-targeted VLDL; VTG, vitellogenin.
Literature Cited
- 1.Agricultural Marketing Service Programs. United States standards, grades, and weight classes for shell eggs, American Marketing Service publication 56, Marketing and Regulatory Programs. Washington, DC: USDA; 2000.
- 2.Wallace BP, Sotherland PR, Spotila JR, Reina RD, Franks BF, Paladino FV. Biotic and abiotic factors affect the nest environment of embryonic leatherback turtles, Dermochelys coriacea. Physiol Biochem Zool. 2004;77:423–32 [DOI] [PubMed] [Google Scholar]
- 3.Chen SE, McMurtry JP, Walzem RL. Overfeeding-induced ovarian dysfunction in broiler breeder hens is associated with lipotoxicity. Poult Sci. 2006;85:70–81 [DOI] [PubMed] [Google Scholar]
- 4.Pan YE, Liu ZC, Chang CJ, Xie YL, Chen CY, Chen CF, Walzem RL, Chen SE. Ceramide accumulation and up-regulation of proinflammatory interleukin-1 exemplify lipotoxicity to mediate declines of reproductive efficacy of broiler hens. Domest Anim Endocrinol. 2012;42:183–94 [DOI] [PubMed] [Google Scholar]
- 5.Walzem RL, Davis PA, Hansen RJ. Overfeeding increases very low density lipoprotein diameter and causes the appearance of a unique lipoprotein particle in association with failed yolk deposition. J Lipid Res. 1994;35:1354–66 [PubMed] [Google Scholar]
- 6.Yu MW, Robinson FE, Charles RG, Weingardt R. Effect of feed allowance during rearing and breeding on female broiler breeders. 2. Ovarian morphology and production. Poult Sci. 1992;71:1750–61 [DOI] [PubMed] [Google Scholar]
- 7.Richards MP, Poch SM, Coon CN, Rosebrough RW, Ashwell CM, McMurtry JP. Feed restriction significantly alters lipogenic gene expression in broiler breeder chickens. J Nutr. 2003;133:707–15 [DOI] [PubMed] [Google Scholar]
- 8.Bruggeman V, Onagbesan O, Vanmontfort D, Berghman L, Verhoeven G, Decuypere E. Effect of long-term food restriction on pituitary sensitivity to cLHRH-I in broiler breeder females. J Reprod Fertil. 1998;114:267–76 [DOI] [PubMed] [Google Scholar]
- 9.Waddington D, Perry MM, Gilbert AB, Hardie MA. Follicular growth and atresia in the ovaries of hens (Gallus domesticus) with diminished egg production rates. J Reprod Fertil. 1985;74:399–405 [DOI] [PubMed] [Google Scholar]
- 10.Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol. 2003;41:1071–7 [DOI] [PubMed] [Google Scholar]
- 11.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7 [DOI] [PubMed] [Google Scholar]
- 12.Hocking PM, Waddington D, Walker MA, Gilbert AB. Control of the development of the ovarian follicular hierarchy in broiler breeder pullets by food restriction during rearing. Br Poult Sci. 1989;30:161–73 [DOI] [PubMed] [Google Scholar]
- 13.Yu MW, Robinson FE, Robblee AR. Effect of feed allowance during rearing and breeding on female broiler breeders. 1. Growth and carcass characteristics. Poult Sci. 1992;71:1739–49 [DOI] [PubMed] [Google Scholar]
- 14.Griffin HD, Perry MM. Exclusion of plasma lipoproteins of intestinal origin from avian egg yolk because of their size. Comp Biochem Physiol B. 1985;82:321–5 [DOI] [PubMed] [Google Scholar]
- 15.Perry MM, Griffin HD, Gilbert AB. The binding of very low density and low density lipoproteins to the plasma membrane of the hen’s oocyte. A morphological study. Exp Cell Res. 1984;151:433–46 [DOI] [PubMed] [Google Scholar]
- 16.Etches RJ. Reproduction in poultry. Wallingford, UK: CAB International; 1996. p. 317.
- 17.USDA-ARS National Nutrient Database for Standard Reference. Nutrient Data Laboratory; 2011 [cited 2013 Aug 8]. Available from: http://ndb.nal.usda.gov/ndb/search/list.
- 18.Williams TD, Reed WL, Walzem RL. Egg size variation: mechanisms and hormonal control. In: Dawson A, Chaturvedi CM, editors. Avian endocrinology. Chennai, India: Narosa Publishing House; 2002. p. 205–18.
- 19.Walzem RL. Lipoproteins and the laying hen: form follows function. Poult Avian Biol Rev. 1996;7:31–64 [Google Scholar]
- 20.Walzem RL, Hansen RJ, Williams DL, Hamilton RL. Estrogen induction of VLDLy assembly in egg-laying hens. J Nutr. 1999;129:467S–72S [DOI] [PubMed] [Google Scholar]
- 21.Wiskocil R, Bensky P, Dower W, Goldberger RF, Gordon JI, Deeley RG. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc Natl Acad Sci USA. 1980;77:4474–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiskocil R, Goldman P, Deeley RG. Cloning and structural characterization of an estrogen-dependent apolipoprotein gene. J Biol Chem. 1981;256:9662–7 [PubMed] [Google Scholar]
- 23.Schneider WJ, Carroll R, Severson DL, Nimpf J. Apolipoprotein VLDL-II inhibits lipolysis of triglyceride-rich lipoproteins in the laying hen. J Lipid Res. 1990;31:507–13 [PubMed] [Google Scholar]
- 24.Griffin H, Grant G, Perry M. Hydrolysis of plasma triacylglycerol-rich lipoproteins from immature and laying hens (Gallus domesticus) by lipoprotein lipase in vitro. Biochem J. 1982;206:647–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyle-Roden E, Walzem RL. Integral apolipoproteins increase surface-located triacylglycerol in intact native apoB-100-containing lipoproteins. J Lipid Res. 2005;46:1624–32 [DOI] [PubMed] [Google Scholar]
- 26.Timmins PA, Poliks B, Banaszak L. The location of bound lipid in the lipovitellin complex. Science. 1992;257:652–5 [DOI] [PubMed] [Google Scholar]
- 27.Takahashi S, Kawarabayasi Y, Nakai T, Sakai J, Yamamoto T. Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci USA. 1992;89:9252–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stifani S, Barber DL, Nimpf J, Schneider WJ. A single chicken oocyte plasma membrane protein mediates uptake of very low density lipoprotein and vitellogenin. Proc Natl Acad Sci USA. 1990;87:1955–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacon WL, Leclercq B, Blum JC. Difference in metabolism of very low density lipoprotein from laying chicken hens in comparison to immature chicken hens. Poult Sci. 1978;57:1675–86 [DOI] [PubMed] [Google Scholar]
- 30.Elkin RG, Zhong Y, Donkin SS, Hengstschlager-Ottnad E, Schneider WJ. Effects of atorvastatin on lipid metabolism in normolipidemic and hereditary hyperlipidemic, non-laying hens. Comp Biochem Physiol B Biochem Mol Biol. 2006;143:319–29 [DOI] [PubMed] [Google Scholar]
- 31.Elkin RG, Zhong Y, Porter RE, Jr, Walzem RL. Validation of a modified PCR-based method for identifying mutant restricted ovulator chickens: substantiation of genotypic classification by phenotypic traits. Poult Sci. 2003;82:517–25 [DOI] [PubMed] [Google Scholar]
- 32.Elkin RG, Bauer R, Schneider WJ. The restricted ovulator chicken strain: an oviparous vertebrate model of reproductive dysfunction caused by a gene defect affecting an oocyte-specific receptor. Anim Reprod Sci. 2012;136:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hummel S, Lynn EG, Osanger A, Hirayama S, Nimpf J, Schneider WJ. Molecular characterization of the first avian LDL receptor: role in sterol metabolism of ovarian follicular cells. J Lipid Res. 2003;44:1633–42 [DOI] [PubMed] [Google Scholar]
- 34.Schneider WJ. Receptor-mediated mechanisms in ovarian follicle and oocyte development. Gen Comp Endocrinol. 2009;163:18–23 [DOI] [PubMed] [Google Scholar]
- 35.Johnson PA. Follicle selection in the avian ovary. Reprod Domest Anim. 2012;47(Suppl 4):283–7 [DOI] [PubMed] [Google Scholar]
- 36.Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92 [DOI] [PubMed] [Google Scholar]
- 37.Brännström M, Enskog A. Leukocyte networks and ovulation. J Reprod Immunol. 2002;57:47–60 [DOI] [PubMed] [Google Scholar]
- 38.Bukulmez O, Arici A. Leukocytes in ovarian function. Hum Reprod Update. 2000;6:1–15 [DOI] [PubMed] [Google Scholar]
- 39.Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10:119–33 [DOI] [PubMed] [Google Scholar]
- 40.Nili H, Kelly WR. Form and function of lacunae in the ovary of the laying hen. Anat Rec. 1996;244:165–74 [DOI] [PubMed] [Google Scholar]
- 41.Gupta SK, Gilbert AB, Walker MA. Histological study of follicular atresia in the ovary of the domestic hen (Gallus domesticus). J Reprod Fertil. 1988;82:219–25 [DOI] [PubMed] [Google Scholar]
- 42.Sundaresan NR, Saxena VK, Sastry KV, Nagarajan K, Jain P, Singh R, Anish D, Ravindra PV, Saxena M, Ahmed KA. Cytokines and chemokines in postovulatory follicle regression of domestic chicken (Gallus gallus domesticus). Dev Comp Immunol. 2008;32:253–64 [DOI] [PubMed] [Google Scholar]
- 43.Barua A, Yoshimura Y, Tamura T. The effects of age and sex steroids on the macrophage population in the ovary of the chicken, Gallus domesticus. J Reprod Fertil. 1998;114:253–8 [DOI] [PubMed] [Google Scholar]
- 44.Barua A, Yoshimura Y, Tamura T. Effects of ageing and oestrogen on the localization of immunoglobulin-containing cells in the chicken ovary. J Reprod Fertil. 1998;114:11–6 [DOI] [PubMed] [Google Scholar]
- 45.Barua A, Yoshimura Y, Tamura T. Localization of macrophages in the ovarian follicles during the follicular growth and postovulatory regression in chicken, Gallus domesticus. Poult Sci. 1998;77:1417–21 [DOI] [PubMed] [Google Scholar]
- 46.Onagbesan OM, Mast J, Goddeeris B, Decuypere E. Effect of TNF-alpha on LH and IGF-I modulated chicken granulosa cell proliferation and progesterone production during follicular development. J Reprod Fertil. 2000;120:433–42 [PubMed] [Google Scholar]
- 47.Cornax I, Walzem RL, Larner C, Macfarlane RD, Klasing KC. Mobilization of ectopic yolk in Gallus gallus domesticus: a novel reverse lipid transport process. J Exp Biol. 2013;216:1949–58 [DOI] [PubMed] [Google Scholar]
- 48.Friedman SL. Mac the knife? Macrophages—the double-edged sword of hepatic fibrosis. J Clin Invest. 2005;115:29–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13:289–312 [DOI] [PubMed] [Google Scholar]
- 50.Ogiwara K, Takano N, Shinohara M, Murakami M, Takahashi T. Gelatinase A and membrane-type matrix metalloproteinases 1 and 2 are responsible for follicle rupture during ovulation in the medaka. Proc Natl Acad Sci USA. 2005;102:8442–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hy-Line International. Hy-Line W-36 performance standards manual. 2 ed. Commercial layers. West Des Moines, IA: Hy-Line International; 2012. p. 22.
- 52. Cobb-Vantress I. Cobb 500 breeder management guide. Siolam Springs, AR: Cobb-Vantress; 2003. p. 42.
- 53.Jaap RG, Muir FV. Erratic oviposition and egg defects in broiler-type pullets. Poult Sci. 1968;47:417–23 [Google Scholar]
- 54.Purdum SE, Didde D. Fats and fatty acids in laying hens. In: Cherian G, Poureslami R, editors. Fats and fatty acid in poultry nutrition and health. Leicestershire, UK: Context Products and Lightning Source; 2012. p. 1–4.
- 55.Yu MW, Robinson FE, Etches RJ. Effect of feed allowance during rearing and breeding on female broiler breeders. 3. Ovarian steroidogenesis. Poult Sci. 1992;71:1762–7 [DOI] [PubMed] [Google Scholar]
- 56.Maurice DV, Jensen LS. Hepatic lipid metabolism in domestic fowl as influenced by dietary cereal. J Nutr. 1979;109:872–82 [DOI] [PubMed] [Google Scholar]
- 57.Polin D, Wolford JH. Various types of diets, sources of energy, and positive energy balance in the induction of fatty liver hemorrhagic syndrome. Poult Sci. 1976;55:325–34 [DOI] [PubMed] [Google Scholar]
- 58.Walzem RL, Simon C, Morishita T, Lowenstine L, Hansen RJ. Fatty liver hemorrhagic syndrome in hens overfed a purified diet. Selected enzyme activities and liver histology in relation to liver hemorrhage and reproductive performance. Poult Sci. 1993;72:1479–91 [DOI] [PubMed] [Google Scholar]
- 59.Barron LG, Walzem RL, Hansen RJ. Plasma lipoprotein changes in hens (Gallus domesticus) during an induced molt. Comp Biochem Physiol B Biochem Mol Biol. 1999;123:9–16 [DOI] [PubMed] [Google Scholar]
- 60.Webster AB. Physiology and behavior of the hen during induced molt. Poult Sci. 2003;82:992–1002 [DOI] [PubMed] [Google Scholar]
- 61.Ruszler PL. Health and husbandry considerations of induced molting. Poult Sci. 1998;77:1789–93 [DOI] [PubMed] [Google Scholar]
- 62.Griffin HD, Guo K, Windsor D, Butterwith SC. Adipose tissue lipogenesis and fat deposition in leaner broiler chickens. J Nutr. 1992;122:363–8 [DOI] [PubMed] [Google Scholar]
- 63.Dupont J, Chen J, Derouet M, Simon J, Leclercq B, Taouis M. Metabolic differences between genetically lean and fat chickens are partly attributed to the alteration of insulin signaling in liver. J Nutr. 1999;129:1937–44 [DOI] [PubMed] [Google Scholar]
- 64.Behr SR, Patsch JR, Forte T, Bensadoun A. Plasma lipoprotein changes resulting from immunologically blocked lipolysis. J Lipid Res. 1981;22:443–51 [PubMed] [Google Scholar]
- 65.Nakanishi S, Yamane K, Kamei N, Nojima H, Okubo M, Kohno N. A protective effect of adiponectin against oxidative stress in Japanese Americans: the association between adiponectin or leptin and urinary isoprostane. Metabolism. 2005;54:194–9 [DOI] [PubMed] [Google Scholar]
- 66.Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med. 2003;254:32–44 [DOI] [PubMed] [Google Scholar]
- 67.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–36 [DOI] [PubMed] [Google Scholar]
- 68.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–31 [DOI] [PubMed] [Google Scholar]
- 69.Zaghari M, Taherkhani R, Honarbakhs H. Effects of progesterone injection on performance, plasma hormones and ovarian morphology of ad libitum and restricted fed broiler breeder hens. Afr J Biotechnol. 2009;8:6481–9 [Google Scholar]
- 70.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–61 [DOI] [PubMed] [Google Scholar]
- 71.Spicer LJ, Francisco CC. The adipose obese gene product, leptin: evidence of a direct inhibitory role in ovarian function. Endocrinology. 1997;138:3374–9 [DOI] [PubMed] [Google Scholar]
- 72.Duggal PS, Van Der Hoek KH, Milner CR, Ryan NK, Armstrong DT, Magoffin DA, Norman RJ. The in vivo and in vitro effects of exogenous leptin on ovulation in the rat. Endocrinology. 2000;141:1971–6 [DOI] [PubMed] [Google Scholar]
- 73.Xie YL, Pan YE, Chang CJ, Tang PC, Huang YF, Walzem RL, Chen SE. Palmitic acid in chicken granulosa cell death-lipotoxic mechanisms mediate reproductive inefficacy of broiler breeder hens. Theriogenology. 2012;78:1917–28 [DOI] [PubMed] [Google Scholar]
- 74.Luheshi GN, Gardner JD, Rushforth DA, Loudon AS, Rothwell NJ. Leptin actions on food intake and body temperature are mediated by IL-1. Proc Natl Acad Sci USA. 1999;96:7047–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santana P, Llanes L, Hernandez I, Gonzalez-Robayna I, Tabraue C, Gonzalez-Reyes J, Quintana J, Estevez F, Ruiz de Galarreta CM, Fanjul LF. Interleukin-1 beta stimulates sphingomyelin hydrolysis in cultured granulosa cells: evidence for a regulatory role of ceramide on progesterone and prostaglandin biosynthesis. Endocrinology. 1996;137:2480–9 [DOI] [PubMed] [Google Scholar]
- 76.Donesky BW, Dias de Moura M, Tedeschi C, Hurwitz A, Adashi EY, Payne DW. Interleukin-1beta inhibits steroidogenic bioactivity in cultured rat ovarian granulosa cells by stimulation of progesterone degradation and inhibition of estrogen formation. Biol Reprod. 1998;58:1108–16 [DOI] [PubMed] [Google Scholar]
- 77.Tobai H, Nishiya I. Nitric oxide mediates inhibitory effect of interleukin-1beta on estrogen production in human granulosa-luteal cells. J Obstet Gynaecol Res. 2001;27:53–9 [DOI] [PubMed] [Google Scholar]
- 78.Guo X, Li H, Xu H, Halim V, Zhang W, Wang H, Ong KT, Woo SL, Walzem RL, Mashek DG, et al. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS One. 2012;7:e39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mu YM, Yanase T, Nishi Y, Tanaka A, Saito M, Jin CH, Mukasa C, Okabe T, Nomura M, Goto K, et al. Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells. Endocrinology. 2001;142:3590–7 [DOI] [PubMed] [Google Scholar]
- 81.Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–45 [DOI] [PubMed] [Google Scholar]
- 82.Benson JD, Bensadoun A, Cohen D. Lipoprotein lipase of ovarian follicles in the domestic chicken (Gallus domesticus) (38537). Proc Soc Exp Biol Med. 1975;148:347–50 [DOI] [PubMed] [Google Scholar]