Figure 1. Schematic of TGFβ production as a latent complex.
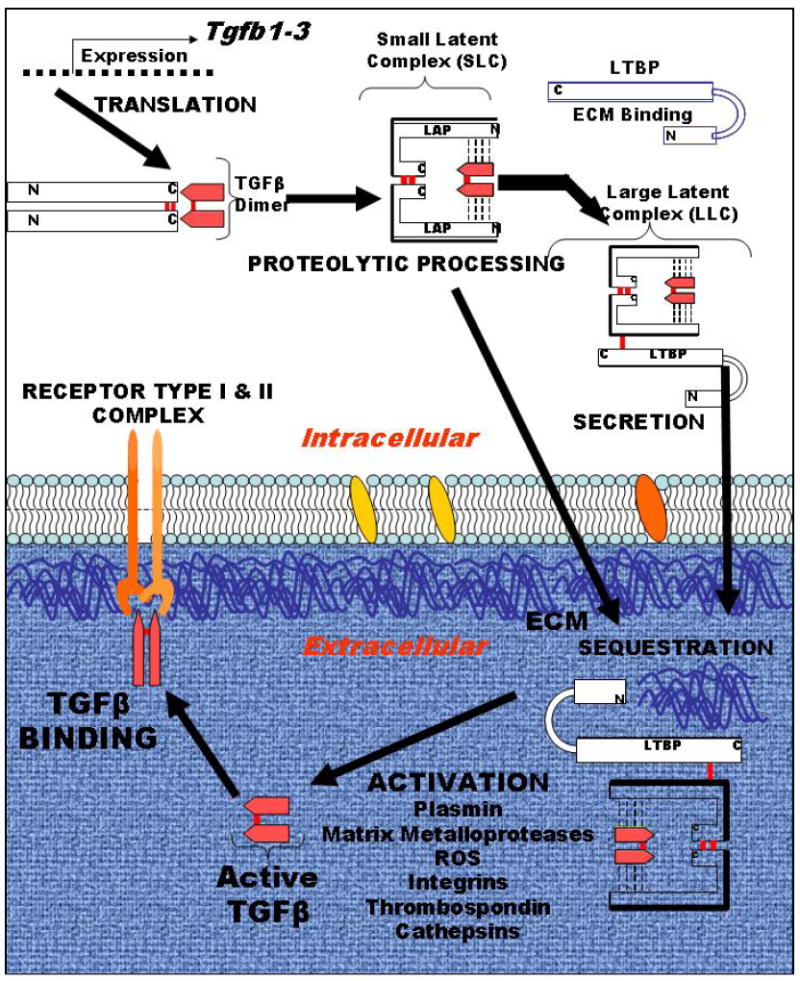
TGFβ 1-3 isoform genes encode a single polypeptide that is processed, cleaved to make a homodimer of the pre-pro peptide, which is designated as latency associated protein (LAP), that is non-covalently bound to TGFβ proper, which is a 24 kD homodimer, which associate in a non-covalent bound complex. LAP acts as a chaperone for proper folding, contains the signal sequence for secretion and sequesters TGFβ until activation. This so-called small latent complex can also form the large latent complex upon covalently binding the latent TGFβ binding proteins (LTBP), which sequester latent TGFβ in the extracellular matrix (ECM). Activation occurs by multiple relatively understudied mechanisms, as listed, to release TGFβ for binding to its receptors, type I and II, which then initiates the signaling cascade.
