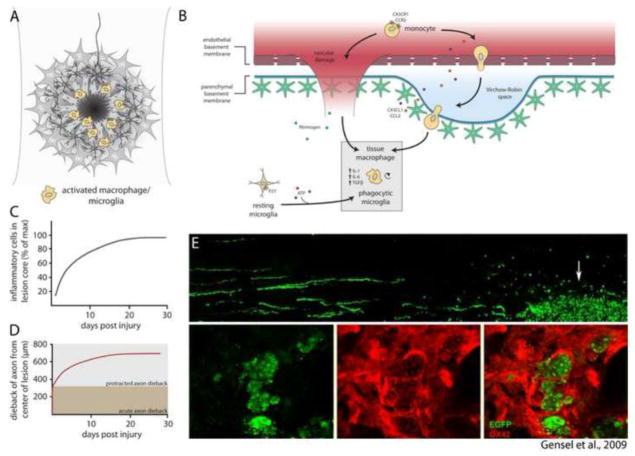
Fig. 2.
Inflammatory processes within the lesion core. (A) After injury, macrophages and microglia accumulate within the lesion core. (B) Recruitment of inflammatory cells occurs by extravasation of leukocytes from damaged blood vessels and migration of resident microglia to sites of CNS injury. Tissue macrophages and phagocytic microglia synthesize a contingent of cytokines that promote inflammation. (C) Accumulation of inflammatory cells within the lesion core reaches peak density by 30 days after injury (data adapted from Horn et al., 2008; Kigerl et al., 2009). (D) Dieback of injured axons occurs in two distinct phases: Acute axonal degeneration occurs via intracellular Ca2+-dependent cysteine proteases, whereas protracted axonal dieback occurs via direct interaction with inflammatory cells. Protracted axonal dieback correlates well with the accumulation of inflammatory cells within the lesion core (C). Data in (D) adapted from Kerschensteiner et al. (2005) and Horn et al. (2008). (E) Data republished from Gensel et al. (2009) with permission from the Society for Neuroscience; permission conveyed through the Copyright Clearance Center, Inc. EGFP labeled dorsal root ganglion neurons were microtransplanted at a site distant to zymosan injection. EGFP+ axons are observed growing toward the site of zymosan injection, where activated OX42+ macrophages (red) are observed engulfing DRG axon fragments.