Abstract
FGF10 is required for embryonic epidermal morphogenesis including brain development, lung morphogenesis, and initiation of limb bud formation. In this study, we investigated the role of FGF10 as a lead induction factor for stem cell differentiation toward urothelial cell. To this end, human multi-potent stem cell in vitro system was employed. Human amniotic fluid stem cells were co-cultured with immortalized bladder cancer lines to induce directed differentiation into urothelial cells. Urothelial markers, uroplakin II, III and cytokeratin 8, were monitored by RT-PCR, immunocytochemistry and western blot analysis. Co-cultured stem cells began to express uroplakin II, III and cytokeratin 8. Targeted FGF10 gene knock down from bladder cancer cells abolished the directed differentiation. In addition, when FGF10 downstream signaling was blocked with the mek inhibitor, the co-culture system lost the capacity to induce urothelial differentiation. Exogenous addition of recombinant FGF10 protein promoted stem cell differentiation into urothelium cell lineage. Together, this report suggests that paracrine FGF10 signaling stimulates the differentiation of human stem cell into urothelial cells. Current study provides insight into the potential role of FGF10 as a lead growth factor for bladder regeneration and its therapeutic application for bladder transplantation.
Keywords: Human amniotic fluid stem cell, Fibroblast growth factor 10, Urothelium, Co-culture, Bladder cancer cells
INTRODUCTION
Pre-clinical and clinical studies with bladder tissue engineering, based on autologous bladder biopsies, have shown promising early results for bladder regeneration (Atala et al. 2006). When normal autologous cells are not available, multi-potent stem cells including the recently described human amniotic fluid stem cells (HAFSC), may be useful as a potentially endless source of versatile cells for bladder tissue engineering purposes (De Coppi et al. 2007, Kim et al. 2007). However, to date, the specific mechanisms that guide bladder cell differentiation from stem cells are poorly understood.
Co-culture of multi-potent stem cells with a target cell population has previously been shown to induce differentiation of the stem cells into specific cell types, including pulmonary epithelium (Van Vranken et al. 2005), retinal cells (Sugie et al. 2005), liver cells (Lange et al. 2005), and neural cells (Kitajima et al. 2005), most likely by specific growth factors released from the target cells during the co-culture. Alternatively, cancer cell co-cultivation has been used as a source of the growth factors required for cellular changes in many cases including co-culturing with lung cancer (Rippon et al. 2008), breast cancer (Selvey et al. 2004), colorectal cancer cells (Koshida et al. 2006) and leukemia cells (Glenjen et al. 2005).
In this report, we describe the stem cell co-culture with bladder cancer cells. Three human bladder cancer cell lines were used for the co-culture, LD605, LD611 and LD627. Multiple apoptosis-associated genes were methylated in both LD605 and LD611 (Fridriech et al. 2004). In LD605 and LD611, one or more vascular endothelial growth factor (VEGF) receptors were expressed (Wu et al. 2003). It was also shown that EphB4 receptor tyrosine kinase was expressed in both LD605 and LD611 (Xia et al. 2006). Interestingly, EphB4 receptor was phosphorylated only in LD605.
FGF family members function in embryonic development, cell growth and morphogenesis. Fibroblast growth factor 10 (FGF10) is a paracrine-acting, epithelial mitogen produced by cells of mesenchymal origin that acts exclusively through a subset of receptors, including fibroblast growth factor receptor 2b (FGFr2b) that is expressed predominantly by epithelial cells. FGF10 signaling plays an important role in bladder urothelial cell proliferation and migration, as FGF10 functions as a mitogen for acceleration of epithelial regeneration after bladder urothelial injury (Bagai et al. 2002). We tested the hypothesis that FGF10/mek signaling plays a role in bladder urothelial differentiation. Current data revealed the potential role of FGF10 signaling for directed differentiation of multi-potent stem cell toward urothelial cell lineages.
MATERIALS AND METHODS
Cell lines and culture
HAFSC were isolated and maintained as previously described (De Coppi et al. 2003). Undifferentiated stem cells were maintained in Chang’s medium (alpha-minimal essential medium 400ml, L-glutamine 5ml, Penicillin/Streptomycin 5ml, Chang Supplements 12.5ml and fetal bovine serum 100ml; Irvine Scientific, Irvine, CA) and incubated at 37°C in a 95% humidified chamber with 5% carbon dioxide. The human bladder cancer cell lines LD605, LD611 and LD627 (a kind gift of Dr. Peter Jones, USC) were grown in Dulbecco’s modified Eagle medium (DMEM) containing 10% heat- inactivated fetal calf serum, penicillin (100 U/ml) and streptomycin (100 μg/ml; Life Technologies, Rockville, MD). Lung adenocarcinoma A549 (a gift of Dr. Wei Ding, Children’s hospital in Los Angeles) was cultured in the same culture medium of DMEM/FBS/Antibiotics. Both bladder cancer and lung cancer cells were incubated at 37°C in a 95% humidified chamber with a 5% carbon dioxide.
Bladder cancer cell -stem cell co-culture
Undifferentiated stem cells and bladder cancer cells were co-cultured indirectly (without contact, but with exchanges of soluble factors) using 0.4 μm pore-sized membrane tissue culture inserts (Becton Dickinson labware, Catalog number 353495, translucent polyethylene terephthalate membrane, Franklin Lakes, NJ) for up to 4 weeks. Cell suspensions containing 3,000 amniotic fluid stem cells were seeded in 24-well tissue culture plates. Bladder cancer cell suspensions containing 3,000 cells were seeded onto the tissue culture inserts, pre-cultured for 16 hours, then placed into the corresponding wells. The cells were co-cultured in DMEM containing 10% heat-inactivated fetal calf serum, penicillin (100 U/ml) and streptomycin, and incubated at 37°C in a 95% humidified chamber with 5% carbon dioxide.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde. Primary antibodies for the bladder markers cytokeratin, uroplakin II, and uroplakin III (Santa Cruz Biotechnology Inc., Santa Cruz, CA), were diluted 1:200 ratio in 1.5% horse serum in PBS (Phosphate buffered saline). Primary antibodies were incubated with the cells for 1 hour at room temperature. Then, secondary antibodies (biotinylated, Vector incorporated, Burlingame, CA) were added and incubated for 30 minutes at room temperature. Chromogenic substrate extravidine- fluorescein (Vector incorporated, Burlingame, CA) was added and incubated for 15 minutes in dark. All of the human stem cell stained DAPI and fluorescence pictures were taken with Leica DFC280 camera (Leica, Wetzlar, Germany) linked to the Leica DM IL microscope (Leica, Wetzlar, Germany).
RT-PCR analysis
To examine gene expression in co-cultured stem cells, RT-PCR was performed using primers for uroplakin III, cytokeratin 8, and FGF-10 genes. Oligonucleotide sequences for uroplakin III are forward primer 5′-AACAACCCCACACTTACCACTG-3′ and reverse primer 5′-GGAGCTTGCTGGAATACACCTC-3′. Oligonucleotide sequences for human cytokeratin 8 (KRT8) are forward primer 5′-GCCTGGTTCGGCCCGCCTGCCTCC-3′ and reverse primer 5′-GGCTGAGGGCTAGGGCTGAGCCTC-3′. Oligonucleotide sequences for Human FGF10 are forward primer 5′-ATGTGGAAATGGATACTGACACATTG-3′ and reverse primer 5′-TACATTTGCCTCCCATTATGCTGCC-3′. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. Primer sequences for GAPDH are forward primer 5′-CCCCAATGTATCCGTTGTGGA-3′ and reverse primer 5′-GCCTGCTTCACCACCTTCTT-3′. Total RNAs were extracted from the co-cultured human stem cells. Then, 1 μg of total RNA was reverse transcribed and amplified using OneStep RT-PCR kit (Qiagen, Valencia, CA). To monitor the FGF10 expression in the human bladder cancer cells, total RNAs were also extracted with RNeasy mini-prep kit (Qiagen, Valencia, CA). One microgram of total RNA was reverse transcribed and amplified with the primers specific to human FGF10 gene. The RT-PCR conditions used were 50°C for 30 minutes, 95°C for 15 minutes, 35 cycles at 94°C for 1 minute, 55°C for 1 minute and 72°C for 1 minute.
Targeted FGF10 knock-down and U0126 – mek inhibitor treatment
FGF10 gene was targeted knocked down by using FGF10 siRNA gene silencer (Santa Cruz Biotechnology; sc-39462), following manufacturer instructions. FGF10 gene was silenced by transfecting siRNAs to 10μM and RT-PCR was performed to confirm gene knock-down. To block FGF-10 signaling downstream, U0126 (Calbiochem, San Diego, CA), a mek inhibitor, was added at a concentration of 20μM to the stem cell culture medium in co-culture system as previously described (Cushing et al. 2008).
Recombinant FGF10 protein addition
Recombinant FGF10 protein (R&D Systems, Minneapolis, MN) was diluted from a stock containing hanks balanced salt solution and bovine serum albumin to a final media concentration of 50, 100, 150 and 200 ng/ml. We observed four different concentration of FGF10 to find the optimal condition for differentiation into urothelium.
RESULTS
Co-cultivation induces early urothelial differentiaton
To determine whether certain cancer cell line can induce the stem cell differentiation into bladder urothelium cells, we performed the stem cell-cancer cell co-culture in vitro. After two weeks of co-culture with bladder cancer cell LD605, approximately 45~50% of the human amniotic fluid stem cells began to change their morphology to the polygonal epithelial structures, while the mono-cultured cells did not show the same morphological changes (Figure 1A). Moreover, the co-cultured stem cells started to express uroplakin III and cytokeratin 8 RNAs from the RT-PCR analysis (Figure 1B). Consistently, western data showed the urothelial-lineage specific protein expressions from the co-culture (Figure 1B). Immunocytochemistry was performed with the human stem cells co-cultured with bladder cancer cell line LD605. Co-cultured stem cells showed positive staining of UPII, UPIII and CK8, while no expression was seen in the mono-culture control (Figure 1C). Our results suggest that certain bladder cancer cell lines possess the differentiation capacity through the mechanisms involved in paracrine signaling.
Figure 1.
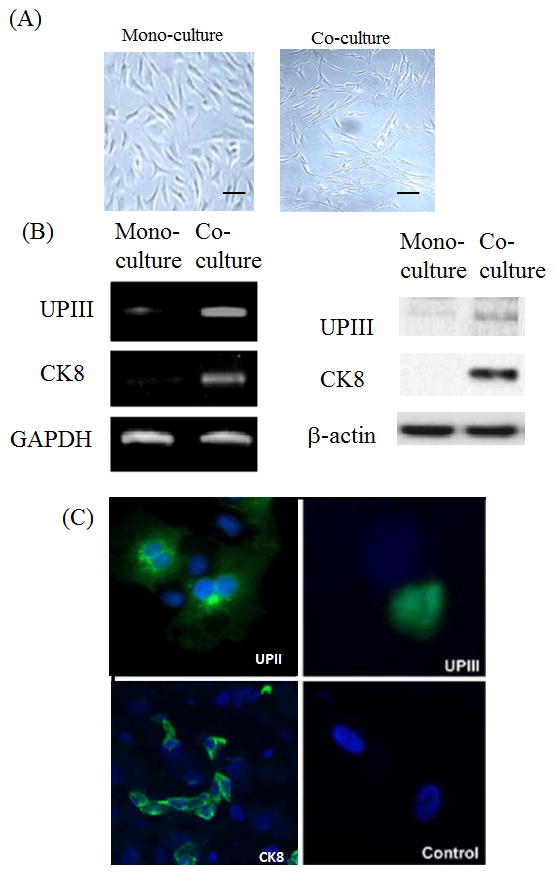
(A) Human amniotic fluid stem cell morphology from the mono-culture and co-culture. Human stem cells were culture in the mono-culture and co-culture systems and morphology was observed after 2 weeks. Scale bar 100 μm. (B) Left panel: RT-PCR was performed with FGF10, uroplakin III and cytokeratin 8 gene primers in stem cells co-cultured with bladder cancer cell line LD605. Right panel: Western blot was performed with the stem cells co-cultured and mono-cultured. FGF10 protein and urothelial markers, UPIII and CK8, were examined on the PAGE analysis. (C) Immunofluorescence of bladder markers uroplakin II, uroplakin III and cytokeratin 8 in the co-culture system. Amniotic fluid stem cells cultured alone (mono-culture) served as a control.
Down-regulation of urothelium markers with FGF10 knock-down
Stem cell co-culturing with a certain bladder cancer cell line LD605 induced a differentiation into bladder cell lineages. This possibly was induced by soluble factor(s) secreted from LD605 cell line. Our next study is to find out what molecular events are responsible for the differentiation. Fibroblast Growth Factor 10 is a mitogen for epithelial regeneration after bladder urothelial injury and its signaling is known to play an important role in bladder urothelial cell proliferation (Bagai et al. 2002). FGF10 is also a critical morphogen involved in many organs’ morphogenesis such as prostate gland (Thomson and Cunha, 1999; Huang et al. 2005), liver growth during embryogenesis (Berg et al. 2007) and early human kidney development (Carev et al. 2008). We hypothesized that FGF10 signaling is involved in the urothelial differentiation of stem cells. Hence we decided to examine FGF10 knock down effects on the stem cell differentiation. FGF10 gene was silenced by the siRNA transfection and confirmed by RT-PCR (Figure 2A). Then, we monitored the human stem cell differentiation from the co-culture by RT-PCR analysis of bladder cell markers (Figure 2B). When FGF10 gene was silenced, the co-cultured stem cells did not express the bladder cell markers, suggesting that FGF10 may be required for the differentiation process. Next, we extended to turn off the FGF10 downstream signaling and observe the directed differentiation.
Figure 2.
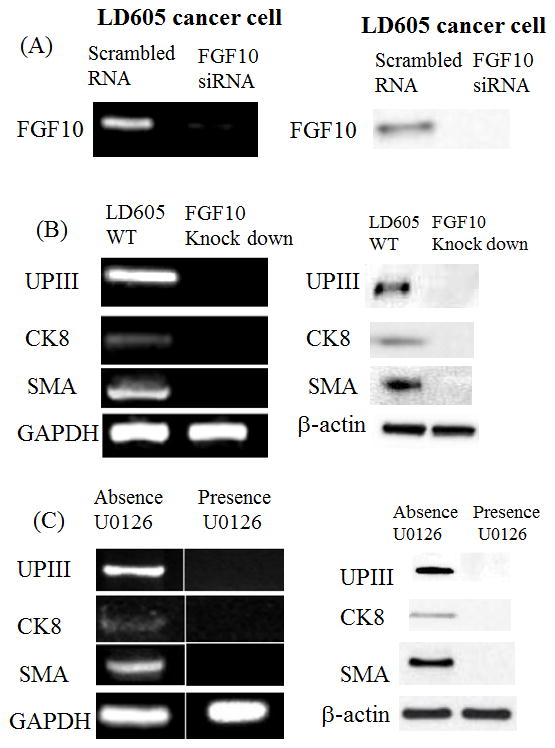
(A) FGF10 gene was targeted knocked-down from the LD605 cancer cell lines. (B) Co-cultured human stem cells were observed for the bladder cell markers with FGF10 knock-down cells. Left panel: RT-PCR results of UP III, CK8 and SMA were presented. Right panel: Western blot data of the same markers were presented. (C) After the addition of U0126 to block the downstream FGF-10 signaling pathway, RT-PCR revealed no expression of the bladder markers uroplakin III and cytokeratin 8, and no expression of smooth muscle actin in the co-culture.
U0126 is the highly selective inhibitor of Mek1 and Mek2 kinase in mitogen activated protein kinase (MAPK) pathway and known to efficiently shut down FGF10 signaling. In the presence of U0126, stem cell/cancer cell co-culture was performed. There was no change in morphology or viability of stem cells with the concentration of 20μM concentration of U0126. We monitored the bladder differentiation markers, UPIII and CK8 and smooth muscle actin (SMA) expression. RT-PCR analysis revealed no expression of the bladder markers from the U0126 present co-culture suggesting FGF10 signaling play a role in stem cell differentiation into bladder lineages (Figure 2C).
FGF10 was expressed in LD605 and FGF10 addition promoted urothelial differentiation
Since only LD605 was able to induce the HAFSC differentiation into bladder cells, we wished to determine whether FGF10 was specifically expressed in LD605. RT-PCR analysis was performed with LD605 and other bladder cancer line LD611. Human amniotic fluid stem cells (SC) did not express FGF10 while LD611 expressed low level of FGF10. LD605 expressed FGF10 clearly (Figure 3A). Lung adenocarcinoma cell line A549 was examined as a control. A549 expressed very low level of FGF10 gene. Our results indicate that FGF10 expressing cancer cell has the differentiation capacity for multi-potent stem cells. This is consistent with the data that revealed FGF10 knock down abrogated the stem cell differentiation induction.
Figure 3.
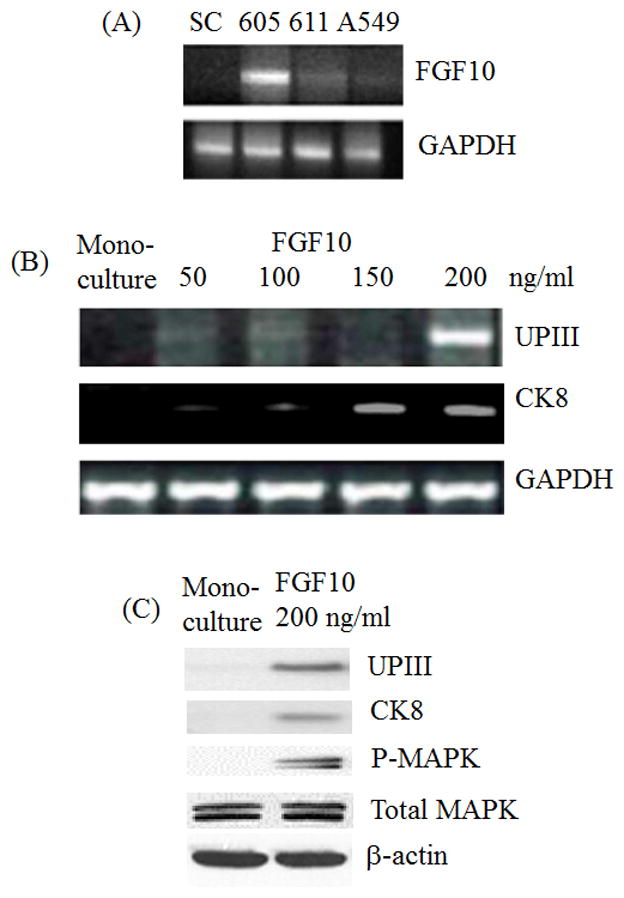
(A) FGF10 overexpression in LD605. RT-PCR analysis was performed with total RNAs extracted from LD605, LD611 and A549 cancer cells. Total RNAs were extracted from HAFSC and lung adenocarcinoma A549 cells. SC stands for human stem cells, 605 stands for human bladder cancer cell line LD605 and 611 stands for human bladder cancer cell line LD611. A549 stands for human lung adenocarcinoma cell line A549. (B) RT-PCR was performed in the absence and presence of recombinant FGF10 protein with the final concentration of 50, 100, 150 and 200 ng/ml. Cytokeratin 8 and uroplakin III expressions were monitored from the monoculture and FGF10 added culture. (C) Western blot was performed with the stem cells mono-cultured and recombinant FGF10 added culture. Uroplakin III, cytokeratin 8, Phospho-MAPK and total MAPK proteins were monitored on the 10% polyacrylamide gel electrophoresis. Upon FGF10 treatment, UPIII and CK8 proteins began to express. Phopho-MAPK also began to express upon FGF10 addition.
To confirm the role of FGF10 in induction, recombinant FGF10 protein was added to the stem cell culture to a final concentration of 50, 100, 150 and 200 ng/ml. Exogenous addition of FGF10 protein induced the urothelial differentiation from the stem cells. UPIII gene was expressed with 200 ng/ml of FGF10 and CK8 was also expressed with 150 ng/ml of FGF (Figure 3B). Urothelial marker proteins, UPIII and CK8, were also expressed in the presence of FGF10 200 ng/ml (Figure 3C). Phospho-MAPK (mitogen-activated protein kinase) was also activated upon FGF10 treatment. These data are pointing FGF10 as a leading induction factor for the stem cell differentiation toward early bladder cell lineage.
DISCUSSION
For patients with end-stage bladder diseases, current bladder replacement techniques utilize intestinal segments, which are associated with significant perioperative morbidity and long-term complications (Gilbert and Hensle, 2005). As an alternative approach, multi-potent stem cells are envisioned as a viable source of cells from which damaged or diseased tissue can be replaced. This report describes a co-culture system in which human amniotic fluid stem cells were induced to differentiate toward the urothelial-specific lineage in vitro by co-culturing with human bladder cancer cells. The co-culture system appears to be a viable system to investigate the urothelial differentiation process, especially since the factors governing this process are currently unknown. There is still possibility that human amniotic stem cells are more prone to induced to bladder lineage as their origin source was amniotic fluid which are associated with urinary bladder location-wise and tissue-wise. It remains to be seen if this specific induction holds true with other stem cells from different origins.
FGF-10 is one factor that apparently plays a role in the urothelial differentiation process. With the bladder cancer cell co-culture system, we have demonstrated the FGF10 expressing bladder cancer cell has the capacity to induce stem cell differentiation into urothelium. This is most likely paracrine effects of the LD605 in the co-culture. The other bladder cancer cell line LD611 and lung adenocarcinoma cell line A549 did not show the directed differentiation capacity (data not shown). The addition of U0126, a downstream inhibitor of mek in the FGF-10 signaling pathway, to the co-culture system inhibited the expression of bladder markers, uroplakin III and cytokeratin 8. This underscores the potential role of the FGF-10 signaling pathway, and specifically the mek pathway, in early urothelial differentiation.
Since bladder cancer cell co-culture system was employed, we wished to check whether the cancer cell co-culture with human stem cells induced the stem cell tumorigenesis. Soft agar colony assay with mono-cultured HAFSC, co-cultured HAFSC showed no differences in growth in the anchorage-independent conditions in soft agar (Personal communications with Dr. Hyung- Gyoo Kang at Children’s hospital in Los Angeles). This indicates that the cancer cell co-culture does not necessarily induce stem cell tumorigenesis in co-culturesystem.
Future experiments will be directed toward further elucidation of the components of these pathways as well as the manipulation of FGF-10 activity to optimize the urothelial differentiation process from the human stem cells.
Acknowledgments
This study was supported by NIH / NIDDK 5K08DK078589-2, an American College of Surgeons Faculty Research Fellowship, and a research grant from the Spina Bifida Association awarded to Chester J. Koh.
References
- Atala A, Bauer S, Soker S, Yoo J, Retik A. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1215–1216. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- Bagai S, Rubio E, Cheng J, Sweet R, Thomas R, Fuchs E. Fibroblast growth factor-10 is a mitogen for urothelial cells. Journal of Biological Chemistry. 2002;277:23828–23837. doi: 10.1074/jbc.M201658200. [DOI] [PubMed] [Google Scholar]
- Berg T, Rountree C, Lee L, Estrada J. Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via beta- catenin activation. Hepatology. 2007;46:1187–1197. doi: 10.1002/hep.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carev D, Saraga M, Saraga-Babic M. Involvement of FGF and BMP family proteins and VEGF in early human kidney development. Histol Histopathol. 2008;23:853–862. doi: 10.14670/HH-23.853. [DOI] [PubMed] [Google Scholar]
- Cushing M, Mariner P, Jo-Tsu L, Sims E, Anseth K. Fibroblast growth factor represses Smad-mediated myofibroblast activation in aortic valvular interstitial cells. FASEB Journal. 2008;22:1769–1777. doi: 10.1096/fj.07-087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coppi P, Bartsch G, Siddiqui M, Xu T, Santos C, Perin L. Isolation of amniotic stem cell lines with potential for therapy. Nature Biotechnology. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Weisenberger D, Cheng J. Detection of methylated apoptosis-associated genes in urine sediments of bladder cancer patients. Clinical Cancer Research. 2004;10:7457–7465. doi: 10.1158/1078-0432.CCR-04-0930. [DOI] [PubMed] [Google Scholar]
- Gilbert S, Hensle T. Metabolic consequences and long-term complications of enterocystoplasty in children: a review. Journal of Urology. 2005;173:1080–1086. doi: 10.1097/01.ju.0000155248.57049.4e. [DOI] [PubMed] [Google Scholar]
- Glenjen N, Hatfield K, Bruserud O. Coculture of native human acute myelogenous leukemia blasts with fibroblasts and osteoblasts results in an increase of vascular endothelial growth factor levels. European Journal of Haematology. 2005;74:24–34. doi: 10.1111/j.1600-0609.2004.00333.x. [DOI] [PubMed] [Google Scholar]
- Huang L, Pu Y, Alam S, Birch L, Prins G. The role of Fgf10 signaling in branching morphogenesis and gene expression of the rat prostate gland: lobe- specific suppression by neonatal estrogens. Dev Biol. 2005;278:396–414. doi: 10.1016/j.ydbio.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee Y, Kim H, Hwang H, Kwon H, Kim S, Cho D, Kang S, You J. Human amniotic fluid-driven stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007;40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima H, Yoshimura S, Kokuzawa J, Kato M, Iwama T, Motohashi T. Culture method for the induction of neurospheres from mouse embryonic stem cells by coculture with PA6 stromal cells. Journal of Neuroscience Research. 2005;80:467–474. doi: 10.1002/jnr.20469. [DOI] [PubMed] [Google Scholar]
- Koshida Y, Kuranami M, Watanabe M. Interaction between stromal fibroblasts and colorectal cancer cells in the expression of vascular endothelial growth factor. Journal of Surgical Research. 2006;134:270–277. doi: 10.1016/j.jss.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Lange C, Bassler P, Lioznov M, Bruns H, Kluth D, Zander A. Liver-specific gene expression in mesenchymal stem cells is induced by liver cells. World Journal of Gastroenterology. 2005;11:4497–4504. doi: 10.3748/wjg.v11.i29.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon H, Lane S, Zin M, Ismail N, Wilson M, Takata M, Bishop A. Embryonic stem cells as a source of pulmonary epithelium in vitro and in vivo. Proc Am Thorac Soc. 2008;5:717–722. doi: 10.1513/pats.200801-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvey S, Haupt L, Thompson E, Matthaei K, Irving M, Griffiths L. Stimulation of MMP-11 (stromelysin-3) expression in mouse fibroblasts by cytokines, collagen and co-culture with human breast cancer cell lines. BMC Cancer. 2004;4:40. doi: 10.1186/1471-2407-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugie Y, Yoshikawa M, Ouji M, Saito K, Moriya K, Ishizaka S. Photoreceptor cells from mouse ES cells by co-culture with chick embryonic retina. Biochemical & Biophysical Research Communications. 2005;332:241–247. doi: 10.1016/j.bbrc.2005.04.125. [DOI] [PubMed] [Google Scholar]
- Thomson A, Cunha G. Prostate growth and development are regulated by FGF10. Development. 1999;126:3693–3701. doi: 10.1242/dev.126.16.3693. [DOI] [PubMed] [Google Scholar]
- Van Vranken B, Romanska H, Polak J, Rippon H, Shannon J, Bishop A. Coculture of embryonic stem cells with pulmonary mesenchyme: a microenvironment that promotes differentiation of pulmonary epithelium. Tissue Engineering. 2005;11:1177–1187. doi: 10.1089/ten.2005.11.1177. [DOI] [PubMed] [Google Scholar]
- Wu W, Shu X, Hovsepyan H, Mosteller R, Broek D. VEGF receptor expression and signaling in human bladder tumors. Oncogene. 2003;22:3361–3370. doi: 10.1038/sj.onc.1206285. [DOI] [PubMed] [Google Scholar]
- Xia G, Kumar S, Stein J. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene. 2006;25:769–780. doi: 10.1038/sj.onc.1209108. [DOI] [PubMed] [Google Scholar]


