Abstract
Patients with multiple myeloma who are refractory or intolerant to both bortezomib and lenalidomide have a poor prognosis. Next-generation therapies carfilzomib and pomalidomide have shown promising activity in this dual refractory population. Here we describe the clinical characteristics and ascertain the effects of carfilzomib and pomalidomide on survival in this patient cohort. We retrospectively reviewed the records of 65 dual refractory/intolerant myeloma patients diagnosed between January 2007 and May 2012 at a single institution. The median overall survival (OS) from the time patients became dual refractory/intolerant was 10.2 months. Patients who received carfilzomib or pomalidomide after they became dual refractory/intolerant had a better OS compared to those who did not (12.6 vs. 6.8 months, p=0.03 by Wilcoxon test). Prospective randomized control trials are needed for confirmation.
Keywords: multiple myeloma, lenalidomide, bortezomib, pomalidomide, carfilzomib
Introduction
Multiple Myeloma (MM) is the second most common hematologic malignancy in the United States [1,2]. Approximately 21,000 new cases and 10,000 deaths were associated with MM in 2012 [1]. In the past decade, the introduction of novel agents, including the immunomodulatory drugs (IMiDs) and proteasome inhibitors, has resulted in significant improvement of response rates, duration of remission, and progression free survival (PFS) [2]. Despite these advances, MM remains incurable, and the majority of patients will relapse or progress on available therapies. Patients refractory to both IMiDs and proteasome inhibitors have a dismal prognosis. Kumar et al reported a PFS of 5 months and overall survival (OS) of 9 months in this dual refractory population; however, their report did not provide detailed characteristics of this highly refractory population [3].
Next-generation IMiDs and proteasome inhibitors are designed to improve target specificity, increase efficacy, and minimize toxicity. Pomalidomide, an IMiD, and carfilzomib, a proteasome inhibitor, are two examples of the next-generation agents. Both have recently gained accelerated approvals from the United States Food and Drug Administration (FDA) in the setting of refractory MM [4,5]. Carfilzomib and pomalidomide have shown promising activity in both refractory and newly diagnosed MM in clinical trials [6-9], but more data and longer follow-up are needed to determine their effects on survival.
In the current study, we define detailed clinical characteristics of MM patients who are dual refractory or intolerant to both bortezomib and lenalidomide. In addition, given that many of our patients had received carfilzomib or pomalidomide on clinical trials, we aim to ascertain the survival effects of carfilzomib and pomalidomide in this patient cohort.
Patients and Methods
Patients followed at the Siteman Cancer Center/Washington University who became dual refractory or intolerant to bortezomib and lenalidomide between January 2007 and May 2012 were identified by reviewing medical records. The primary objectives of the study were to define in detail the characteristics of patients who became refractory or intolerant to both bortezomib and lenalidomide and to investigate the effects of next-generation therapies carfilzomib and pomalidomide on the OS in this refractory population. The second objective was to identify factors associated with survival. The study was approved by the Institutional Review Board of Washington University. The requirement for informed consent was waived because the measurements performed in the study were part of routine clinical care and confidentiality was maintained. This study was not supported by any funding sources.
Eligible patients were 18 years of age or older with demonstrated progression on or intolerance to both bortezomib and lenalidomide. To represent “real world practice,” no patients were excluded based on ECOG (Eastern Cooperative Oncology Group) performance status or the amount of measurable disease. Detailed data collection forms were used for unified collection of clinical and laboratory data at the time of MM diagnosis and when patients became dual refractory/intolerant. We identified and recorded the type, time of initiation and discontinuation, and reasons for discontinuation of each myeloma treatment regimen.
As per published consensus criteria [10], dual refractory or intolerant disease was defined as disease progressive on bortezomib and lenalidomide or disease that relapsed within 60 days of stopping these treatments, or if patients could not tolerate either or both drugs due to toxicity. Patients could have been refractory or intolerant to these agents either in combination or sequentially. We defined time zero (T0) as the time when patients became dual refractory or intolerant to both bortezomib and lenalidomide. Responses were evaluated according to the IMWG (International Myeloma Working Group) criteria [10]. Overall survival after T0 was defined as the duration between T0 and the time of death. Patients known to be alive on the cut-off date for data analysis were censored on the date of the last encounter. Time to next treatment was calculated from the time of the first therapy after T0 to the time of the second therapy after T0.
Demographics and disease characteristics were summarized by descriptive statistics. We performed univariate and multivariate analyses by Cox regression to identify predictors associated with OS in this population. OS was estimated using Kaplan-Meier method and comparisons were made by Wilcoxon test. All analyses were based on a two sided p-value of 0.05. Analysis was done using SAS version 9.3.
Results
Patient and disease characteristics
A total of 65 patients followed at the Siteman Cancer Center/Washington University who became dual refractory or intolerant to bortezomib and lenalidomide between January 2007 and May 2012 were identified. Table I summarizes patient demographics at initial diagnosis and at T0. The median age at the diagnosis of MM was 58 years (range: 34-77 years). Sixty-three percent (N=41) were male, 78% (N=51) Caucasian, 20% (N=13) African American, and 2% (N=1) Asian. The median age at T0 was 62 years (range: 39-79 years). The median interval from diagnosis to T0 was 39 months (range: 2-160 months). The median number of therapies that patients received prior to T0 was 5 (range: 1-11). The median time to next treatment (TNT) was 3.5 months. Six patients did not receive any therapy after T0 because they rapidly succumbed to the disease (with a median survival after T0 of 1.2 months).
Table I.
Patient characteristics at diagnosis and T0.
| Variable | N/n (%) |
|---|---|
| Diagnosis | |
|
| |
| Age, years, median (range) | 58 (34-77) |
| Gender | |
| Male | 41/65 (63%) |
| Female | 24/65 (37%) |
| Race | |
| Caucasian | 51/65 (78%) |
| African American | 13/65 (20%) |
| Asian | 1/65 (2%) |
| International Staging system | |
| I | 18/46 (39%) |
| II | 15/46 (33%) |
| III | 13/46 (28%) |
|
| |
| T0 | |
|
| |
| Age, years, median (range) | 62 (39-79) |
| Time from diagnosis, months, median (range) | 39 (2-160) |
| Lines of prior therapy, median (range) | 5 (1-11) |
| Serum monoclonal protein heavy chain | |
| IgG | 37/59 (63%) |
| IgA | 17/59 (29%) |
| Negative | 5/59 (8%) |
| Serum monoclonal protein light chain | |
| Kappa | 34/60 (57%) |
| Lambda | 21/60 (35%) |
| Negative | 5/60 (8%) |
Detailed disease characteristics at diagnosis and T0 are listed in Table II. At T0, 72% of patients (47/65) had advanced lytic bone disease (defined as more than three lytic lesions), and 28% (18/64) had poor bone marrow reserve (defined as neutrophils < 1000/mm3 or platelets < 75,000/mm3). Twenty-four percent (15/62) had high-risk cytogenetics (including hypodiploidy, del 13, del 17p, t(4;14), or complex cytogenetics). Thirty-one percent (18/58) of patients had oligosecretory myeloma (defined as serum M-protein < 1g/dL and urine M-protein < 200mg/24hrs) at T0, compared to only 9% (6/65) at diagnosis. Six percent (4/65) had extramedullary disease (defined as presence of masses that have not risen from any bone) at T0, compared to none at diagnosis.
Table II.
Disease characteristics at diagnosis and T0.
| Variable | Diagnosis N/n (%) |
T0 N/n (%) |
|---|---|---|
| Advanced lytic lesions1 | N/A | 47/65 (72%) |
| Oligosecretory disease2 | 6/65 (9%) | 18/58 (31%) |
| Poor bone marrow reserve3 | N/A | 18/64 (28%) |
| High risk cytogenetics or FISH4 | N/A | 15/62 (24%) |
| Extramedullary disease5 | 0/65 (0%) | 4/65 (6%) |
Advanced lytic lesions referred to more than 3 bony lytic lesions.
Oligosecretory disease was defined as serum M-protein < 1g/dL and urine M-protein < 200mg/24hrs.
Poor bone marrow reserve was defined as neutrophils < 1000/mm3 or platelets < 75,000/mm3.
High risk cytogenetics and FISH included hypodiploidy, del 13, del 17p, t(4;14), or complex cytogenetics.
Extramedullary disease was defined as masses that have not risen from any bone. Abbreviations: N/A- not available. FISH- Fluorescence In Situ Hybridization.
Therapies pre- and post-T0
Table III summarizes the treatments patients received before and after T0. Patients received a median of 5 (range: 1-11) lines of therapies prior to T0. In addition to bortezomib and lenalidomide, other common treatments included: thalidomide (63%), anthracyclines (46%), and alkylating agents (37%). Forty-eight (74%) patients had autologous stem cell transplantation (SCT) and none underwent allogeneic SCT prior to T0. After T0, patients received a median of 2 (range: 0-6) lines of therapies, including alkylating agents (55%), pomalidomide (23%), carfilzomib (17%), and anthracyclines (14%). Nine (14%) patients underwent SCT after T0, 5 autologous and 4 allogeneic.
Table III.
Overview of treatments (including chemotherapy and stem cell transplantation) patients received prior to and after T0 (n=65).
| Treatment | Pre T0 N (%) |
Post T0 N (%) |
|---|---|---|
| Median (range) of lines of therapy | 5 (1-11) | 2 (0-6) |
| Bortezomib in any combination | 65 (100%) | 20 (31%) |
| Lenalidomide in any combination | 65 (100%) | 15 (23%) |
| VRD | 20 (31%) | 5 (8%) |
| Thalidomide | 41 (63%) | 2 (3%) |
| Anthracyclines | 30 (46%) | 9 (14%) |
| Doxil | 24 (37%) | 7 (11%) |
| Doxorubicin | 6 (9%) | 2 (3%) |
| Alkylators | 24 (37%) | 36 (55%) |
| Melphalan (conventional dose, excluding transplant) | 12 (18%) | 7 (11%) |
| Cyclophosphamide | 14 (22%) | 28 (43%) |
| Bendamustine | 3 (5%) | 21 (32%) |
| DCEP | 1 (2%) | 7 (11%) |
| M2 | 0 | 5 (8%) |
| Carfilzomib or pomalidomide | 10 (15%) | 22 (34%) |
| Carfilzomib | 9 (14%) | 11 (17%) |
| Pomalidomide | 2 (3%) | 15 (23%) |
| Elotuzumab | 1 (2%) | 2 (3%) |
| Other investigational agents | 14 (22%) | 13 (20%) |
| Stem cell transplantation | 48 (74%) | 9 (14%) |
| Autologous transplant | 48 (74%) | 5 (8%) |
| Allogeneic transplant | 0 | 4 (6%) |
Abbreviations: VRD- bortezomib, lenalidomide, and dexamethasone; DCEP- dexamethasone, cyclophosphamide, etoposide, and cisplatin; M2- vincristine, carmustine, cyclophosphamide, melphalan, and prednisone.
Survival outcomes
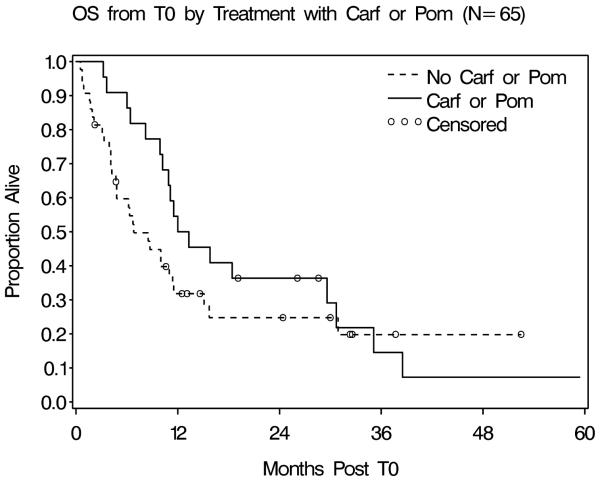
After a mean follow up of 13.5 months, 23% (15/65) of patients were alive. The median OS from T0 was 10.2 months (range: 0.5-59.4 months). Patients who received carfilzomib or pomalidomide after T0 had an improved OS and proportionally fewer early deaths compared to those who did not (p=0.03 by Wilcoxon test, Figure 1). The median survival of the carfilzomib/pomalidomide group was 12.6 months (95% confidence interval [CI] 9.9-29.6 months), compared with 6.8 months (95% CI 4.7-11.4 months) for the group without carfilzomib/pomalidomide. The estimated 12 month OS was 50% (95% CI 28.2-68.4%) in the carfilzomib/pomalidomide group, compared to 31.8% (95% CI 18.2-46.2%) in the group without carfilzomib/pomalidomide.
Figure 1.
Patients who received carfilzomib or pomalidomide after T0 had an improved median overall survival (12.6 months) compared to those without carfilzomib or pomalidomide (6.8 months) (p=0.03 by Wilcoxon test). Figure footnote:Abbreviations: OS- overall survival; Carf- carfilzomib; Pom- pomalidomide.
Factors predictive of poor survival
Univariate analysis identified several factors associated with poor survival, including ISS stage III at diagnosis, advanced lytic lesions at T0, low hemoglobin at T0, low albumin at T0, elevated free light chain at T0, and VRD (bortezomib, lenalidomide, and dexamethasone) prior to T0 (Table IV). Our multivariate analysis model identified four risk factors associated with poor survival: lack of carfilzomib or pomalidomide after T0, low hemoglobin at T0, receiving VRD prior to T0, and advanced lytic lesions at T0 (Table V). Patients who received carfilzomib or pomalidomide had approximately 50% reduction in the risk of death compared to patients who received neither (hazard ratio [HR] 0.52, 95% CI 0.27-0.99, p=0.047). Greater hemoglobin at T0 was associated with a lower hazard of death. The risk of death decreased by 30% with each unit increase in hemoglobin (HR 0.71, 95% CI 0.58-0.86, p=0.0004). Patients who received VRD prior to T0 had poorer survival (HR 2.28, 95% CI 1.19-4.37, p=0.013). The presence of advanced lytic lesions at T0 was associated with a 2.5 times higher risk of death (HR 2.52, 95% CI 1.20-5.29, p=0.014).
Table IV.
Univariate analysis for overall survival
| Variable | n/N (%) | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|---|
| At diagnosis | ||||
| Age ≥ 60 | 24/65 (37%) | 1.60 | 0.88-2.92 | 0.13 |
| Male gender | 41/65 (63%) | 0.95 | 0.52-1.71 | 0.85 |
| Caucasian | 51/65 (78%) | 1.41 | 0.70-2.84 | 0.34 |
| ISS stage III | 13/46 (28%) | 2.99 | 1.42-6.32 | 0.004 |
| At T0 | ||||
| Age ≥ 60 | 38/65 (58%) | 1.09 | 0.61-1.95 | 0.77 |
| Advanced lytic lesions |
47/65 (42%) | 2.30 | 1.13-4.69 | 0.02 |
| Hemoglobin (per unit increase) |
- | 0.79 | 0.65-0.95 | 0.01 |
| Albumin (per unit increase) |
- | 0.48 | 0.27-0.85 | 0.01 |
| Elevated free light chains |
26/53 (49%) | 2.12 | 1.10-4.06 | 0.02 |
| VRD prior to T0 | 20/65 (31%) | 1.96 | 1.07-3.61 | 0.03 |
Abbreviations: CI- confidence interval; ISS- International Staging System; VRD- bortezomib.
Table V.
Predictors of poor survival by multivariate logistic regression
| Variable | Hazard Ratio | 95% Confidence Interval |
p Value |
|---|---|---|---|
| Carf or Pom after T0 (Yes vs. No) |
0.52 | 0.27-0.99 | 0.047 |
| Hemoglobin at T0 (g/dL) (per unit increase) |
0.71 | 0.58-0.86 | 0.0004 |
| VRD prior to T0 (Yes vs. No) |
2.28 | 1.19-4.37 | 0.013 |
| Advanced lytic lesions at T0 (Yes vs. No) |
2.52 | 1.20-5.29 | 0.014 |
Abbreviations: Carf- carfilzomib; Pom- pomalidomide; VRD- bortezomib, lenalidomide, and dexamethasone.
Discussion
In this study, we demonstrate that patients who are dual refractory or intolerant to both bortezomib and lenalidomide have a poor prognosis with a median OS of 10.2 months. They have a high incidence of advanced lytic lesions, oligosecretory phenotype, poor marrow reserve, high-risk cytogenetics, and extramedullary disease. Treatment of dual refractory/intolerant patients with carfilzomib or pomalidomide is associated with an improved survival in this non-randomized retrospective cohort.
Despite the small sample size and the limitations of a single-institution study, our results are comparable to the multicenter IMWG (International Myeloma Working Group) experience [3]. Our patients had a median age of 62 years and received a median of 5 lines of therapies prior to T0, while patients in the IMWG study had a median age of 62.5 years and received a median of 4 treatments. The IMWG study included patients refractory to bortezomib and either refractory or intolerant to one of the IMiDs (thalidomide or lenalidomide). Many of the patients in the IMWG study were not yet refractory or intolerant to lenalidomide at T0. In contrast, our patients were refractory or intolerant to both bortezomib and lenalidomide. Since prospective trials have shown that patients refractory to thalidomide could have a response rate of 40-45% to lenalidomide [11-13], we believe that our patients may have had a more refractory disease.
We identified several important disease characteristics in these refractory patients, which suggest that the disease phenotype and clinical characteristics of the patients evolve over the disease course. Poor marrow reserve (neutrophils < 1000/mm3 or platelets < 75,000/mm3) was found in 28% of patients at T0, and myelosuppressive treatments are likely to be poorly tolerated in these patients. Twenty-four percent of patients had high risk FISH (Fluorescence In Situ Hybridization) or cytogenetics (including hypodiploidy, del 13, del 17p, t(4;14), or complex cytogenetics) at T0, which are known to be associated with more aggressive disease [14]. Larson et al. reported a high incidence of oligosecretory phenotype in a cohort of MM patients who progressed beyond 3 treatment regimens (49% vs. 9% in newly diagnosed MM) [15]. Our study confirmed the increased incidence of oligosecretory phenotype: 31% post-T0 compared to 9% upon diagnosis. Additionally, 7.5% of treatment emergent extramedullary disease was identified in a cohort of relapsed/refractory MM patients treated at Mayo Clinic, most of whom had received novel agents (100% patients had received IMiDs and 78% had received bortezomib) [16]. Our study showed a similar result, with 6% of extramedullary disease emerging at T0, while no extramedullary disease was identified at diagnosis.
Our median OS of 10.2 months was comparable to the 9 months in the IMWG study. We hypothesize that the comparable survival, despite possibly heavier pretreatment, resulted from the benefits of next-generation therapies carfilzomib and pomalidomide. Most of the trials conducted thus far with pomalidomide and carfilzomib have been single arm phase II studies; therefore, the survival benefit of pomalidomide and carfilzomib can only be inferred from comparison with historical data (Table VI). The MM-003 trial did demonstrate an OS advantage for pomalidomide when compared to high dose dexamethasone [17]. Though not a randomized study, we demonstrated a survival advantage associated with these next-generation agents in the setting of chemotherapy (all but six patients in our comparison arm received chemotherapy). Given the nature of the retrospective study, data on performance status could not be reliably ascertained from medical records and therefore could be a confounding factor. Ongoing prospective trials will help in the assessment of the survival benefits for these next-generation agents.
Table VI.
Comparisons of overall response rates and overall survivals from studies of dual refractory or intolerant patients
| Study | Setting | N | Regimen | ORR (≥PR) |
Median OS (95% CI), months |
|---|---|---|---|---|---|
| No carfilzomib or pomalidomide | |||||
|
| |||||
| Kumar et al. 2012 [3] |
Refractory to bortezomib and refractory or intolerant to IMiD |
286 | Physician’s choice | 24%* | 9 (7-11) |
| Dimopoulos et al. 2012 [17] (MM- 003) |
Refractory or intolerant to bortezomib or lenalidomide |
153 | Dexamethasone 40mg days 1- 4, 9-12, 17-20 |
N/A | 8.5 |
| Current study | Dual refractory or intolerant |
43 | Physician’s choice | N/A | 6.8 (4.7-11.4) |
|
| |||||
| With carfilzomib or pomalidomide | |||||
|
| |||||
| Lacy et al. 2011[16] |
Dual refractory | 35 | Pomalidomide 2mg daily Dexamethasone 40mg weekly |
26% | 78% survival at 6 months+ |
| Lacy et al. 2011[16] |
Dual refractory | 35 | Pomalidomide 4mg daily Dexamethasone 40mg weekly |
29% | 67% survival at 6 months‡ |
| Vij et al. 2012 [20] (MM-002) |
Dual refractory | 69 | Pomalidomide 4mg 21/28 days Dexamethasone 40mg weekly |
28% | 13.5 |
| Dimopoulos et al. 2012 [17] (MM- 003) |
Refractory or intolerant to bortezomib or lenalidomide |
302 | Pomalidomide 4mg 21/28 days Dexamethasone 40mg weekly |
N/A | Not reached¶ |
| Leleu et al. 2013 [9] (IFM 2009-02) |
Dual refractory | 64 | Pomalidomide 4mg 21/28 days or 4mg daily Dexamethasone 40mg weekly |
31% | 13.8 (9-16) |
| Siegel et al. 2012 [6] (PX-171-003- A1) |
Dual refractory or intolerant |
214 | Carfilzomib | 20.1% | 13.2 (10.6- 16.6) |
| Current study | Dual refractory or intolerant |
22 | Physician’s choice Carfilzomib or Pomalidomide |
N/A | 12.6 (9.9-29.6) |
Best response rate to first regimen after dual refractory.
Median OS was not reached after a median follow up of 9.7 months
Median OS was not reached after a median follow up of 6.6 months
Median follow-up was 18 weeks.
Abbreviations: ORR- overall response rate; PR- partial responses; OS- overall survival; CI- confidence interval; IMiD- immunomodulatory drugs; N/A- not available.
Similar to our study, the IMWG study identified high β-2 microglobulin (>5.5mg/L) and low albumin (<3.5mg/dL) as predictors for poor survival in the dual refractory population [3]. We identified additional risk factors that are associated with poor survival: lack of carfilzomib or pomalidomide after T0, low hemoglobin at T0, VRD prior to T0, and advanced lytic lesions at T0. Low hemoglobin and advanced lytic lesions at T0 indicate more advanced disease, so it is not surprising that they are associated with poorer survival. VRD is one of the most effective regimens for multiple myeloma [18,19]. However, our data demonstrated a poorer overall survival in patients who received VRD as either induction or salvage therapy prior to T0. Since our study was retrospective in nature with a relative small sample size, we could not exclude the possible confounding effects that adverse demographic and disease characteristics that led to treating physicians’ choosing VRD for therapy resulted in reduced survival in these patients. Our observation should be evaluated in larger prospective dataset. Lastly, the OS benefit with next-generation agents carfilzomib and pomalidomide found by Kaplan-Meier method was confirmed by multivariate analysis showing that patients who received carfilzomib or pomalidomide after T0 had a significantly decreased risk of mortality (HR=0.52).
In conclusion, patients who are dual refractory or intolerant to bortezomib and lenalidomide have a poor prognosis and a high incidence of advanced lytic lesions, oligosecretory phenotype, poor bone marrow reserve, high-risk cytogenetics, and extramedullary disease. This information is crucial for designing future clinical trials for this population of patients. The use of next-generation agents such as carfilzomib and pomalidomide after T0 is associated with approximately 50% reduction in mortality. Prospective clinical trials are needed to explore this further.
Footnotes
Conflicts of Interest Dr. Ravi Vij received honoraria from Celgene, Onyx, and Millennium, research funding from Onyx and Celgene, and is on the Speakers Bureau for Celgene, Millennium, and Teva.
Dr. Stockerl-Goldstein is on the Speakers Bureau for Celgene and Millennium.
The remaining authors declare no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA [Accessed 1/10/2013];Carfilzomib. 2012 < http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm312945.htm>.
- 5.FDA [Accessed 2/11/2013];FDA approves Pomalyst for advanced multiple myeloma. 2013 < http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm338895.htm>.
- 6.Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vij R, Siegel DS, Jagannath S, et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br J Haematol. 2012;158:739–748. doi: 10.1111/j.1365-2141.2012.09232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacy MQ, Allred JB, Gertz MA, et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood. 2011;118:2970–2975. doi: 10.1182/blood-2011-04-348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leleu X, Attal M, Arnulf B, et al. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009-02. Blood. 2013;121:1968–1975. doi: 10.1182/blood-2012-09-452375. [DOI] [PubMed] [Google Scholar]
- 10.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 12.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Dimopoulos MA, Chen C, et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008;112:4445–4451. doi: 10.1182/blood-2008-02-141614. [DOI] [PubMed] [Google Scholar]
- 14.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109:3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 15.Larson D, Kyle RA, Rajkumar SV. Prevalence and monitoring of oligosecretory myeloma. N Engl J Med. 2012;367:580–581. doi: 10.1056/NEJMc1206740. [DOI] [PubMed] [Google Scholar]
- 16.Short KD, Rajkumar SV, Larson D, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25:906–908. doi: 10.1038/leu.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimopoulos MA, Lacy MQ, Moreau P, et al. Pomalidomide in combination with low-dose dexamethasone: Demonstrates a significant progression free survival and overall survival advantage, in relapsed/refractory MM: A phase 3, multicenter, randomized, open-label study [abstract] Blood. 2012;120 Abstract LBA-6. [Google Scholar]
- 18.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson KC, Jagannath S, Jakubowiak AJ, et al. Lenalidomide, bortezomib, and dexamethasone in relapsed/refractory multiple myeloma (MM): Encouraging outcomes and tolerability in a phase II study [abstract] J Clin Oncol. 2009;27(Supplement) Abstract 8536. [Google Scholar]
- 20.Vij R, Richardson PG, Jagannath S, et al. Pomalidomide with or without low-dose dexamethasone in patients with relapsed/refractory multiple myeloma: outcomes in patients refractory to lenalidomide and bortezomib [abstract] J Clin Oncol. 2012;30(Supplement) Abstract 8016. [Google Scholar]