Abstract
Objective
High-normal blood pressure (BP) increases the risk of cardiovascular (CV) disease. The mechanisms underlying this increased risk are not clear. Sympathetic activation appears to be a potential mechanism linking high-normal BP to CV disease. This study examined whether high-normal BP compared to optimal BP is linked to sympathoexcitation at rest and/or during laboratory stressors.
Methods
Heart rate (HR), BP and muscle sympathetic nerve activity (MSNA) were obtained at rest and during stress tests (sustained hand grip and mental stress) in 18 subjects (15 males and 3 females) with high-normal BP (systolic BP of 130 to 139 mm Hg, diastolic BP of 85 to 89 mm Hg, or both) and in 12 subjects (10 males and 2 females) with optimal BP (<120/80 mm Hg) matched for age (34±3 years in both groups) and body mass index (25±2 kg/m2 in both groups).
Results
Despite the higher resting BP levels, MSNA was higher in subjects with high-normal BP than in the optimal BP group (26±3 vs 18±2 bursts/min, P<0.05). During sustained hand grip, MSNA increased by 37±14% in high-normal BP group compared with an increase of 49±15% in optimal BP group (P=0.55). Changes during mental stress were 50±28% and 37±12%, respectively (P=0.73). There were no significant differences in SBP responses to handgrip and mental stress between the high-normal and optimal BP groups. Baseline HR and chronotropic responses to stress tests were comparable between the two groups.
Conclusion
In comparison to optimal BP, high-normal BP is associated with increased resting MSNA, but normal neural and circulatory responses to stress tests. These findings suggest that tonic activation of the sympathetic nervous system may precede overt arterial hypertensionand contribute to an excess risk of CV disease in subjects with high-normal BP.
Keywords: heart rate, high-normal blood pressure, hypertension, laboratory stressors, sympathetic nerve activity
INTRODUCTION
Both cardiovascular (CV) morbidity and mortality are strongly related to both systolic and diastolic blood pressure (BP). Prospective longitudinal studies have demonstrated that the CV risk rises as BP increases from a threshold of 115/75 mm Hg (1). The Framingham Heart Study has shown that the risk of future major CV events in subjects with high-normal BP (SBP between 130 and 139 mmHg, and/or DBP between 85 and 89 mm Hg) is two-fold greater than in those with optimal BP (<120/80 mm Hg) (2). The mechanisms underlying this increased risk are not entirely understood, and several blood pressure-independent factors might be implicated.
Microneurographic studies provided strong evidence of increased muscle sympathetic activity (MSNA) in established hypertension (3–5). The contribution of sympathetic activation to the earlier grades of BP elevation is less clear, and prior studies have reported conflicting findings (6–12). These studies focused primarily on patients with so called “borderline” hypertension which is not recognized by the current hypertension guidelines. The study evaluating MSNA in subjects with high-normal BP was limited solely to the resting condition (12). Whether high-normal BP is associated with potentiated sympathetic and hemodynamic responses to stressors remains unknown. We therefore examined pressor, cardiac and sympathetic responses to mental and physical stressors in subjects with high-normal BP compared to individuals with optimal BP.
METHODS
Subjects
We studied 18 subjects (15 males and 3 females) with high-normal BP (systolic pressure of 130 to 139 mm Hg, diastolic pressure of 85 to 89 mm Hg, or both) and 12 subjects (10 males and 2 females) with optimal BP (<120/80 mmHg) matched for age (34±3 years in both groups) and body mass index (25±2 kg/m2 in both groups). All subjects were Caucasian recruited from the same single geographic region (Gdansk community). Office-seated BP was defined as an average of six measurements during two separate visits. None of the subjects was taking any medication nor had any history of chronic diseases. The studies were approved by the Institutional Review Board on Human Investigation and written informed consent was obtained.
Measurements
HR was recorded concurrently from surface electrodes using a 12 lead switch box system (Dual Bio Amp, ADInstruments, Ltd. Oxford, United Kingdom). BP was measured continuously by the Finometer Medical System (FMS B.V., Amsterdam, the Netherlands). Respiration was measured by respiratory belt transducer (ADInstruments, Ltd. Oxford, United Kingdom). Sympathetic nerve activity to the muscle vascular bed was recorded continuously by obtaining multi-unit recordings of efferent postganglionic sympathetic nerve activity using microneurography (662C-3 Nerve Traffic Analysis System, Bioengineering of Iowa University, USA). A tungsten active microelectrode (UNA 40F2S, FHC Inc., Bowdoinham, ME, USA) was inserted directly into the muscle fascicle of the peroneal nerve posterior to the fibular head. A reference electrode was positioned into the muscle 2–3 cm away from the recording electrode. The neural signals were amplified, filtered, rectified, and integrated to obtain a voltage display of sympathetic nerve activity. The inter-individual variability of MSNA assessed as the coefficient of variation (CV), defined as the standard deviation of the repeated measure divided by the average of the two measurements (visit 1 and visit 2) in our laboratory is 4.18±0.98. The following correlation was observed between visit 1 and visit 2 (R2=0.97).
Protocol and procedures
All studies were carried out in the morning after a standard light breakfast. All subjects were asked to avoid smoking for at least 12 hours prior to each study and abstain from alcohol for at least 48 hours. Subjects were studied in the supine position. HR, BP, respiration and MSNA were measured at rest and during physical and mental stressors (sustained hand grip and mental arithmetic).
After 15 minutes of rest, baseline measurements were obtained over ten minutes. Hand grip and mental stress tests were then conducted with a 15-minute interval between each stressor. The isometric handgrip test was performed by asking the subject to sustain a handgrip of 30% of their maximum voluntary contraction for 3 minutes using a dynamometer. The mental stress test involved asking the subject to do serial subtractions as fast as possible for 3 minutes.
Data Analyses
Tracings of ECG, blood pressure, respiration and MSNA were recorded with the PowerLab data acquisition system (AD Instruments; Ltd. Oxford, United Kingdom). The amplitude of each burst was determined and muscle sympathetic activity was calculated as burst frequency (bursts/min) and as burst incidence (bursts/100 heartbeats) and multiplied by mean burst amplitude and expressed as units/min. Changes in integrated MSNA allowed an evaluation of intra-subject changes in sympathetic traffic during the same recording session. A random code was attributed to all recordings and all data analyses were performed completely blinded to the identity of the subject. Muscle sympathetic bursts were identified by a single experienced investigator.
Results are expressed as means ± SEM. Comparisons between the two groups were made by an unpaired Student’s t-test. A P<0.05 was considered significant.
RESULTS
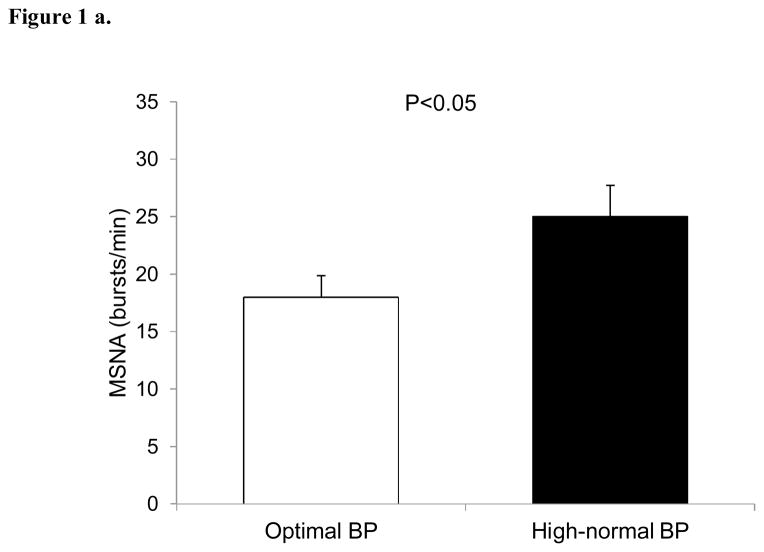
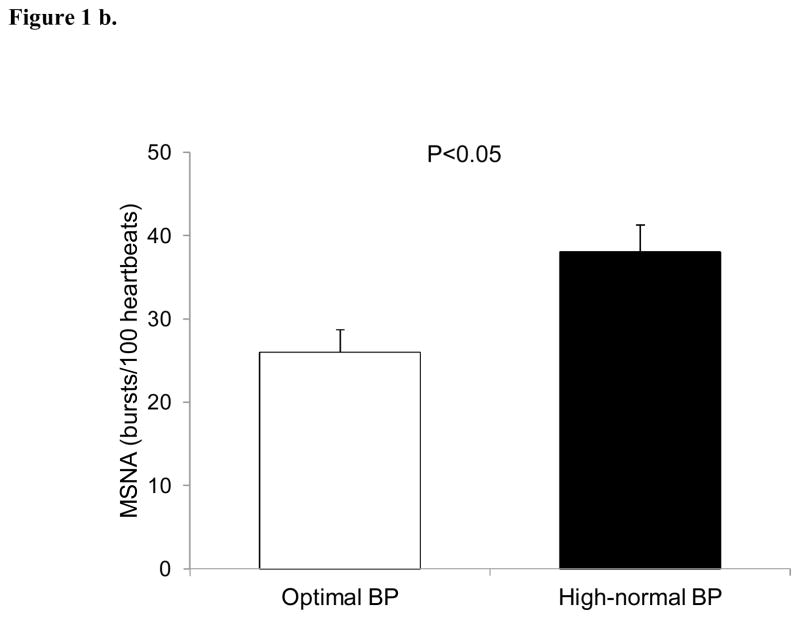
Resting MSNA was elevated in subjects with high-normal BP compared to the optimal BP (26±3 vs 18±2 bursts/min, P<0.05) (Figure 1a). Similar results were obtained when MSNA was expressed as burst incidence (38±3 vs 26±3 bursts/100 heartbeats, P<0.05), respectively (Figure 1b).
Figure 1.
Baseline muscle sympathetic nerve activity (MSNA) in subjects with optimal and high-normal blood pressure (BP) as expressed as bursts/min (Fig. 1a) and bursts/100 heartbeats (Fig. 2a). Values are means ± SEM.
Baseline HR was similar between the two groups (Table 1).
Table 1.
Resting blood pressure and heart rate in subjects with optimal and high-normal blood pressure (BP).
| Measurement | Optimal BP | High-normal BP | P |
|---|---|---|---|
| SBP (mmHg) | 116±2 | 138±2 | <0.001 |
| DBP (mmHg) | 74±1 | 83±2 | 0.002 |
| HR (bpm) | 70±3 | 67±3 | 0.54 |
Values are means ± SEM. SBP indicates systolic blood pressure; DBP diastolic blood pressure; HR, heart rate.
BP, HR and MSNA significantly increased in response to mental stress and hand grip in both groups, but this increase was not different in the high-normal group when compared to the optimal BP group (Table 2).
Table 2.
Systolic blood pressure, heart rate and MSNA responses to handgrip and mental stress tests in subjects with optimal and high-normal blood pressure (BP).
| Measurement | Optimal BP | High-normal BP | P |
|---|---|---|---|
| Handgrip test | |||
| Δ SBP (mmHg) | 9.2±2.7 | 9.6±1.8 | 0.90 |
| Δ HR (bpm) | 4.0±0.8 | 6.1±1.2 | 0.14 |
| Δ MSNA (%) | 49±15 | 37±14 | 0.55 |
| Mental stress | |||
| Δ SBP (mmHg) | 5.8±2.1 | 3.5±1.5 | 0.37 |
| Δ HR (bpm) | 6.0±1.8 | 8.3±2.1 | 0.45 |
| Δ MSNA (%) | 37±12 | 50±28 | 0.73 |
Values are means ± SEM. SBP indicates systolic blood pressure; HR, heart rate; MSNA, muscle sympathetic nerve activity.
In the high-normal BP group, baseline MSNA was not related to MSNA changes during handgrip (r=0.12; P=0.63) or mental stress (r=0.10; P=0.70).
DISCUSSION
The major finding of the present study is that high-normal BP is associated with increased resting sympathetic activity, and normal sympathetic and hemodynamic responses to physical and mental stress tests. These findings support the concept that tonic or resting activation of the sympathetic nervous system may precede overt arterial hypertension and contribute to an excess risk of CV disease in subjects with high-normal BP.
It has been suggested that balance between tonic levels of resting sympathetic activity and adrenergic responsiveness importantly contributes to BP regulation, and its disruption may be a factor in the development of hypertension (13). Therefore, we decided to evaluate both resting measurements and stress-induced changes in sympathetic activity using microneurography which remains the golden standard technique for assessment of autonomic CV regulation. Additionally, we were specifically interested in CV and sympathoadrenal reactivity to mental stress which has been previously shown to be more reproducible than responses to the cold pressor test (14). Our data indicate that in the individuals with high-normal BP, the magnitude of MSNA response to acute stress is not predicted by resting levels of sympathetic activation.
A prior study based on measurements of plasma catecholamines reported that young healthy men with elevated screening BP are characterized by increased sympathetic activity (15). Importantly, norepinephrine and epinephrine concentrations during mental arithmetic tests have been shown to predict future BP elevation (16). Earlier studies using microneurography have reported conflicting results regarding sympathetic activity at rest in subjects with early stages of BP elevation, with some studies reporting normal (6–9) or increased (10–12) MSNA in subjects with high-normal BP or “borderline” hypertension. Conflicting results have also been reported in studies examining responses to acute stress. Elevated BP has been associated with either increased (11), normal (9) or decreased (17) MSNA responses to various types of stressors.
Our findings of higher resting MSNA in subjects with high-normal BP are in line with data from Greenwood et al. (12). However, this study was solely restricted to resting assessment without an evaluation of sympathetic responses to laboratory stressors. We evaluated acute sympathetic responsiveness to both physical and mental stress. The responses to mental stress have been implicated in the pathogenesis of hypertension and CV disease (18,19). Our findings are not consistent with the “pressor reactor” hypothesis, suggesting that the link between high-normal BP and CV risk is unlikely to be mediated via potentiation of acute sympathetic and hemodynamic responses to physical and mental challenges. These findings do not speak against the compelling link between chronic stress and hypertension. Our data seems to indicate that this relationship may be mediated through tonic activation of the sympathetic nervous system, but not enhanced acute sympathetic responsiveness.
Elevated HR has been implicated in the development of hypertension and CV disease (20). In our study, baseline HR was not dissimilar between the two groups despite increased MSNA in subjects with high-normal BP. These findings suggest that HR cannot serve as a proxy of sympathetic nervous activity in middle-aged subjects with mild elevation of BP. However, it is important to consider that an increase in BP will induce lower levels of HR and MSNA. Therefore, we are likely to understand the magnitude of chronotropic and sympathetic drive in the high-normal BP group, since both heart rate and MSNA in these subjects would be increased further if BP was reduced to the level observed in the optimal BP group.
Potential limitations of our study include the relatively small number of subjects that may be criticized as unpowered. However, all subjects were comprehensively evaluated for resting BP level and were matched for age and BMI to exclude the effects of aging and excessive weight on resting sympathetic activity. Secondly, we have not performed other measurements of sympathetic tone including plasma catecholamine and noradrenaline spillover. However, reproducibility and sensitivity of plasma catecholamine is poor compared to MSNA (21). While direct measurements of MSNA from the peroneal nerve reflects approximately 20% of the total body sympathetic activity (22) and is closely related to renal noradrenaline spillover (23). Third, given the lack of agreement between neurohumoral, neurophysiological and spectral analysis of low-frequency heart rate in different conditions and disease (24), we have not included data regarding spectral analysis of heart rate variability.
In conclusion, high-normal BP is characterized by tonic activation of the sympathetic nervous system, but not enhanced acute sympathetic responsiveness. Chronic sympathetic overactivity may substantially contribute to development of sustained BP elevation and to an excess risk of CV complications in subjects with high-normal BP. While our findings applied to the individuals with high-normal BP, this may also be relevant to understanding neural circulatory mechanisms in subjects with pre-hypertension.
Acknowledgments
Source of Funding
This study was supported by Research Fellowship Grant from the European Society of Hypertension, and by the Foundation for Polish Science TEAM/2008-2/5 and MISTRZ 8/2008 grants. D. H. has been supported by a Research Fellowship from the Foundation for Polish Science KOLUMB/2010-1. T. K. is supported by the grant of IGA of Ministry of Health (No. NS 10098-4/2008), and by the European Regional Development Fund - Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123). V.S. is supported by NIH grant HL114676 and by the grant from the Czech Ministry of Health No. NS 10098-4/2008, and by European Regional Development Fund - Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123).
Footnotes
Disclosures
None.
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Prospective Studies Collaboration Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, et al. Impact of high normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–7. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 3.Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiologica Scandinavica. 2003;177:275–284. doi: 10.1046/j.1365-201X.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- 4.Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54:690–697. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- 5.Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA. Relationship between central sympathetic activity and stages of human hypertension. Am J Hypertens. 2004;17:217–22. doi: 10.1016/j.amjhyper.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Rea RF, Hamdan M. Baroreflex control of muscle sympathetic nerve activity in borderline hypertension. Circulation. 1990;82:856–862. doi: 10.1161/01.cir.82.3.856. [DOI] [PubMed] [Google Scholar]
- 7.Floras JS, Senn BL. Absence of post exercise hypotension and sympathoinhibition in normal subjects: additional evidence for increased sympathetic outflow in borderline hypertension. Can J Cardiol. 1991;7:253–258. [PubMed] [Google Scholar]
- 8.Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension. 1988;11:608–612. doi: 10.1161/01.hyp.11.6.608. [DOI] [PubMed] [Google Scholar]
- 9.Schobel HP, Heusser K, Schmieder RE, Veelken R, Fischer T, Luft FC. Evidence against elevated sympathetic vasoconstrictor activity in borderline hypertension. J Am Soc Nephrol. 1998;9:1581–7. doi: 10.1681/ASN.V991581. [DOI] [PubMed] [Google Scholar]
- 10.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans: evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 11.Matsukawa T, Gotoh E, Uneda S, Miyajima E, Shionoiri H, Tochikubo O, Ishii M. Augmented sympathetic nerve activity in response to stressors in young borderline hypertensive men. Acta Physiol Scand. 1991;141:157–65. doi: 10.1111/j.1748-1716.1991.tb09064.x. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood JP, Stoker JB, Mary DA. Single-unit sympathetic discharge: quantitative assessment in human hypertensive disease. Circulation. 1999;100:1305–10. doi: 10.1161/01.cir.100.12.1305. [DOI] [PubMed] [Google Scholar]
- 13.Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006;572:821–7. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassellund SS, Flaa A, Sandvik L, Kjeldsen SE, Rostrup M. Long-term stability of cardiovascular and catecholamine responses to stress tests: an 18-year follow-up study. Hypertension. 2010;55:131–6. doi: 10.1161/HYPERTENSIONAHA.109.143164. [DOI] [PubMed] [Google Scholar]
- 15.Fossum E, Høieggen A, Reims HM, Moan A, Rostrup M, Eide I, Kjeldsen SE. High screening blood pressure is related to sympathetic nervous system activity and insulin resistance in healthy young men. Blood Press. 2004;13:89–94. doi: 10.1080/08037050310031008. [DOI] [PubMed] [Google Scholar]
- 16.Flaa A, Eide IK, Kjeldsen SE, Rostrup M. Sympathoadrenal stress reactivity is a predictor of future blood pressure: an 18-year follow-up study. Hypertension. 2008;52:336–41. doi: 10.1161/HYPERTENSIONAHA.108.111625. [DOI] [PubMed] [Google Scholar]
- 17.Lambert EA, Schlaich MP. Reduced sympathoneural responses to the cold pressor test in individuals with essential hypertension and in those genetically predisposed to hypertension. No support for the “pressor reactor” hypothesis of hypertension development. Am J Hypertens. 2004;17:863–8. doi: 10.1016/j.amjhyper.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Lambert EA, Lambert GW. Stress and its role in sympathetic nervous system activation in hypertension and the metabolic syndrome. Curr Hypertens Rep. 2011;13:244–8. doi: 10.1007/s11906-011-0186-y. [DOI] [PubMed] [Google Scholar]
- 19.Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Dawood T, Kaye D, Barton D, Pier C, Guo L, Brenchley C, Jennings G, Lambert E. Chronic mental stress is a cause of essential hypertension: presence of biological markers of stress. Clin Exp Pharmacol Physiol. 2008;35:498–502. doi: 10.1111/j.1440-1681.2008.04904.x. [DOI] [PubMed] [Google Scholar]
- 20.Palatini P. Role of elevated heart rate in the development of cardiovascular disease in hypertension. Hypertension. 2011;58:745–50. doi: 10.1161/HYPERTENSIONAHA.111.173104. [DOI] [PubMed] [Google Scholar]
- 21.Grassi G, Bolla G, Seravalle G, Turri C, Lanfranchi A, Mancia G. Comparison between reproducibility and sensitivity of muscle sympathetic nerve traffic and plasma noradrenaline in man. Clin Sci (Lond) 1997;92:285–289. doi: 10.1042/cs0920285. [DOI] [PubMed] [Google Scholar]
- 22.Esler M, Jennings G, Leonard P, Sacharias N, Burke F, Johns J, Blombery P. Contribution of individual organs to total noradrenaline release in humans. Acta Physiol Scand Suppl. 1984;527:11–16. [PubMed] [Google Scholar]
- 23.Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491 ( Pt 3):881–887. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–34. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]