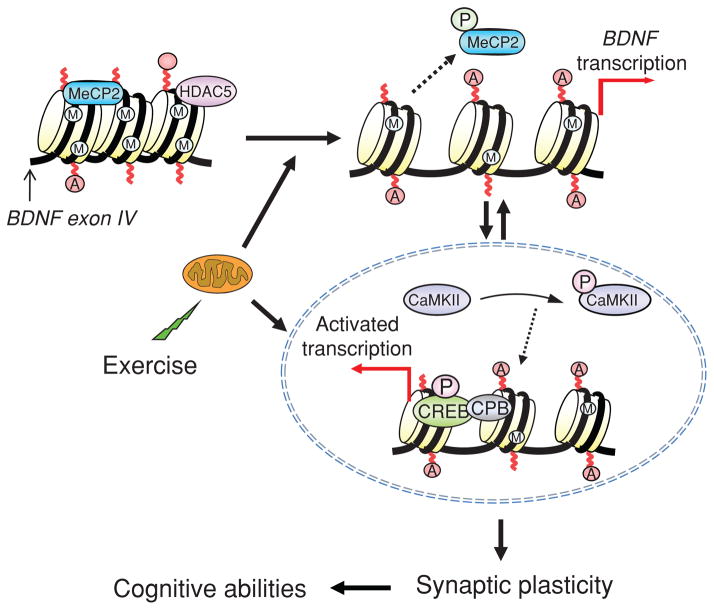
Figure 13.
Proposed mechanism by which exercise impacts synaptic plasticity and cognitive abilities by engaging aspects of epigenetic regulation. As discussed in the text, changes in energy metabolism may be an important mediator for the effects of exercise on synaptic plasticity, in a process engaging mechanisms of epigenetic regulation. Exercise promotes DNA demethylation in BDNF promoter IV, involving phosphorylation of methyl CpG binding protein 2 (MeCP2), and acetylation of histone H3. These events may result in dissociation of MeCP2 and chromatin remodeling events leading to BDNF gene transcription. The effects of exercise on brain-derived neurotrophic factor (BDNF) regulation may also involve the action of histone deacetylases (HDACs) such as HDAC5 implicated in the regulation of BDNF gene (Tsankova et al., 2006). Exercise elevates the activated stages of calcium/calmodulin-dependent protein kinase II (p-CaMKII) and cAMP response element binding protein (p-CREB), which in turn can contribute to regulate BDNF transcription, as well as participate in the signaling events by which BDNF influences synaptic plasticity and cognitive abilities. The impact of exercise on the remodeling of chromatin containing the BDNF gene emphasizes the importance of exercise on the control of gene transcription in the context of brain function and plasticity. Reprinted, with permission, from reference (75), p. 388.