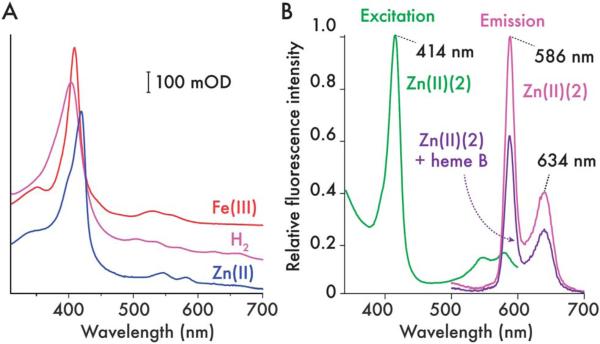
Fig. 7.
Generation of a covalently-appended, light-sensitive cofactor in 2. (A) UV/visible spectra of ferric (Fe(III) (2), red), demetallated (H2(2), pink) and zinc(II) (Zn(II) (2), blue) 2 (5 μM protein, 20 mM phosphate, 100 mM KCl, pH 7.5). Iron is removed from the porphyrin with HF–pyridine solution, then after purification of demetallated 2 (H2(2)), Zn2+ can be inserted to create a covalently bound, photosensitive cofactor. (B) Fluorescence excitation (green) and emission (magenta) spectra of Zn(II) (2) (900 nM, 20 mM CHES, 150 mM KCl. pH 9.0). Emission spectra were recorded with an excitation wavelength of 420 nm in both heme-free and heme-bound Zn(II) (2) and normalized to the Zn(II) (2) maximum at 586 nm.