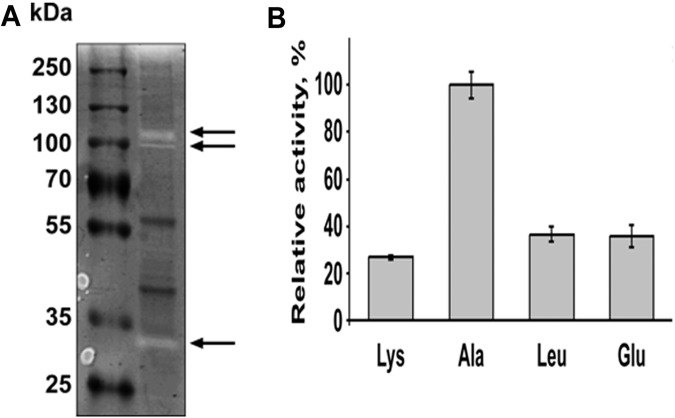
FIG 4 .
OMV display protease activity. (A) Ten micrograms of OMV proteins was loaded on 10% Tris-glycine gel with 0.1% gelatin as the substrate. Following separation at denaturing conditions, proteins were renatured and then incubated at 37°C for 2 days to allow substrate cleavage. Colloidal Coomassie brilliant blue G-250 was used to stain the gel. Clear bands marked by the arrows indicate the digestion of gelatin by proteases. (B) Peptidase activity of B. fragilis vesicles was tested using different p-nitroanilide-linked amino acids. Ten micrograms of purified OMV proteins was incubated with different substrates for 1 h at 37°C. Activities were determined by measuring the absorbance of the released p-nitroanilide at 405 nm. All activities are represented relative to the alanine-peptidase activity. All experiments were done in triplicates.