Figure 6. Foxp3 modulation of class I promoter activity depends on its DNA binding domain.
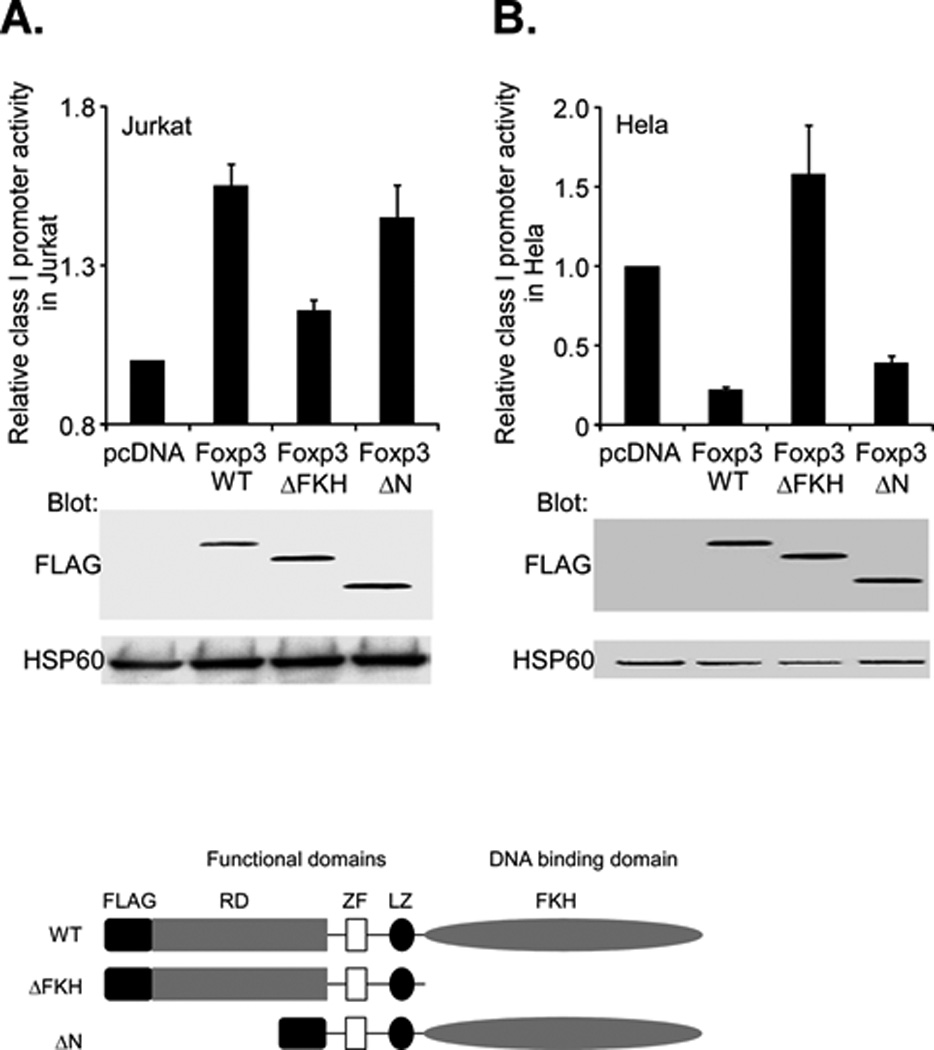
A. Jurkat T cells were cotransfected with the class I WT promoter (1.0µg) and either 2.0 µg of Foxp3 full length (Foxp3-WT), a C-terminal truncation of FKH domain (ΔFKH) or an N-terminal deletion of the proline rich domain (ΔN). Relative promoter activity, as assessed by luciferase activity (upper histogram) was determined. The activity of the ΔFKH construct was significantly less (p<4×10-5) than WT. The activity of the ΔN construct was not significantly different than WT (p=0.13) Expression of Foxp3 constructs was measured by western blotting with an anti-Flag antibody (middle) and compared with the internal control, HSP60 (bottom). Schematic diagrams of the wild type (WT) Foxp3 protein and the derivative truncations are shown below.
B. Hela cells were cotransfected with the class I WT promoter (2.0µg) and either 1.0 µg of Foxp3 full length (Foxp3-WT), a C-terminal truncation of FKH domain (ΔFKH) or an N-terminal deletion of the proline rich domain (ΔN). Relative promoter activity, as assessed by luciferase activity (upper histogram) was determined. Expression of Foxp3 constructs was measured by western blotting with an anti-Flag antibody (middle) and compared with the internal control, HSP60 (bottom).
