Figure 7. Class I deficiency reduces Treg function in vitro and in vivo.
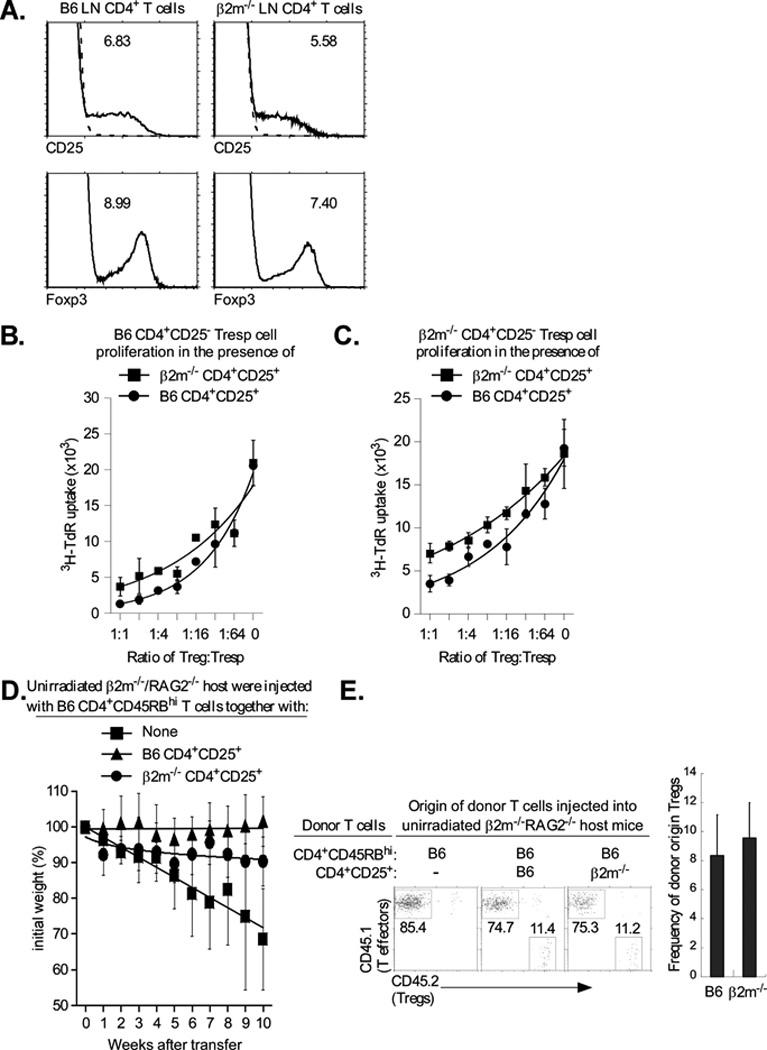
A. MHC class I is not required to generate Tregs. Lymph node T cells were stained for CD25 and Foxp3, as described in Material and Methods. Top panels display CD25 surface staining of gated CD4+ LNT cells and the numbers indicate the percentage of CD4+ T cells that are CD4+CD25+ (dashed lines represent negative control staining). Bottom panels display intracellular Foxp3 staining of gated CD4+ LNT cells and the numbers indicate the percentage of CD4+ T cells that are Foxp3+. Data are representative of three independent experiments.
B., C. MHC class I expression is required for efficient Treg function. CD4+CD25− LN Tconv cells from normal B6 mice (B.) or β2m−/− mice (C.) were cultured with purified CD4+CD25+ Tregs of mice from the two different origins and stimulated to proliferate by anti-CD3 (1µg/ml) and APC. Proliferation was measured by 3H-thymidine incorporation and mean cpm ± SD of triplicate wells are shown. The proliferation levels of B6 and β2m−/− Tregs, alone, were 108±23 and 110±7.2. Data are representative of 3 independent experiments and graphed with best fit curves. The IC50 for the β2m−/− and B6 Tregs was 1:6 and 1:21, respectively with β2m−/− responders.
D. Five week old β2m−/−/RAG-2−/− mice were injected either with 4×105 CD4+CD45RBhigh (CD45.1+) T cells alone, or together with 4×105 purified CD4+CD25+ (CD45.2+) Tregs from either B6 or β2m−/− mice. Tregs of B6 origin were used as a positive control. Percentage change from initial body weight of the recipients was monitored over time. β 2m−/−/RAG-2−/− mice were used as host instead of RAG-2−/− mice which rejected the transferred β 2m−/− Tregs, possibly by NK-mediated killing of class I deficient T cells (data not shown). Each data point represents between 4 and 5 β 2m−/−/RAG-2−/− recipient mice. Data are graphed with best fit curves.
E. β2m−/−/RAG-2−/− mice were injected either with 4×105 CD4+CD45RBhigh (CD45.1+) T cells alone, or together with 4×105 purified CD4+CD25+ (CD45.2+) Tregs from either B6 or β 2m−/− mice. Five weeks later, mice were sacrificed and their mesenteric LNs were stained for the relative proportions of CD4+CD45RBhigh effectors and CD4+CD25+ Tregs. Four or five β2m−/−/RAG-2−/− recipient mice per group were used.
