Abstract
Background:
Urinary tract infection remains one of the most common infections, both in the community and in the hospital. The causative pathogen profile varies from region to region, but Escherichia coli (E. coli) remains the most common causative pathogen. The sensitivity of uropathogens to different drugs varies in different areas, and changes with time. This necessitates periodic studies of the causative uropathogens and their antibiotic sensitivity pattern.
Aim:
To investigate the profile of common uropathogens and assess their antibiotic sensitivity patterns to commonly used antimicrobial agents.
Materials and Methods:
Analysis of all urine specimens submitted for culture and sensitivity was carried out over a 1-year period in a tertiary care hospital in North West India. Urine culture was done by a semi-quantitative method. Antibiotic sensitivity was done on bacterial isolates according to the Clinical and Laboratory Standards Institute CLSI guidelines for disc diffusion susceptibility test. Data was analysed for significance using Chi square test.
Results:
Of a total of 6348 urine specimens received over the 1-year study period, 41.8% (2653) of the urine samples were culture positive. The most common bacterial isolate was E. coli (45.7%, 1103/2412), followed by Coagulase-negative Staphylococci (18.6%, 449/2412) and Klebsiella species (8.3%, 199/2412). The Candida species’ isolation rate was 10.3% (277/2689). The uropathogens displayed a very high level of resistance to fluroquinolones 70.3% (1084/1542)[Inpatient Department (IPD) – 70.5%(572/812), Outpatient Department (OPD) – 70.2%(512/730)] and cephalosporins 75.1%(1158/1542)[IPD – 73.8%(599/812), OPD – 76.6%(559/730)], whereas resistance to nitrofurantoin 19.8%(305/1542) [ IPD – 23.9%, OPD – 15.2%(111/730)], amikacin 32.4%(573/1769) [IPD – 36.1%(235/934), OPD – 28.1%(235/835)] and cephoperazone + sulbactam combination 22%(349/1583) [IPD – 26.2%(244/914), OPD – 15.8%(105/669)] was low.
Conclusion:
Empiric selection of antimicrobial agents should be based on the antibiotic sensitivity pattern of the uropathogens prevalent in that area, which is derived from epidemiological studies carried out in that environment.
Keywords: Antibiotic resistance, Urinary tract infections, Uropathogens
Introduction
Urinary tract infection (UTI) represents one of the most common diseases encountered in medical practice today, occurring from the neonate to the geriatric age group.[1] It is also the most common nosocomial infection, accounting for up to 40% of all nosocomial infections.[2] UTI is particularly responsible for discomfort in elderly patients, representing a risk of bacteremia, septic shock, respiratory distress syndrome and death.[3] Even though several different microorganisms can cause UTI, including protozoan parasites, fungi and viruses, bacteria are the major causative organisms. They account for more than 95% of UTI cases.[4] Bacteria causing UTI are generally of fecal origin.[5,6] Among the bacteria, Escherichia coli (E. coli) is the most common etiological agent, accounting for 75-90% of UTI in both outpatients and inpatients.[7] Complicated UTI exhibits a broader bacterial spectrum as the cause of infection.[7]
In almost all cases of UTI, empirical antimicrobial treatment is initiated before the laboratory results of urine culture and sensitivity are available. Thus, antibiotic resistance may increase in uropathogens due to frequent misuse of antibiotics.[7] In addition, the extensive use of antibiotics, for infections outside the urinary tract, would alter the antibiotic susceptibility pattern of the intestinal bacteria that are generally implicated as uropathogens.[6] Increasing antimicrobial resistance complicates uncomplicated UTI treatment by increasing patient morbidity, costs of reassessment and retreatment and use of broader-spectrum antibiotics.[8] Patterns of antibiotic resistance in a wide variety of pathogenic organisms vary even over short periods of time. Periodic evaluation of antibacterial activity is needed to update this information.[9] For effective treatment and control of UTI in a particular area/hospital, a good knowledge of the antibiotic sensitivity pattern of the causative agents in that area/hospital is of ultimate importance.[6] Furthermore, baseline estimates of the magnitude of the problem and the extent of antimicrobial resistance among the nosocomial pathogens are the minimum essential prerequisites for any hospital infection control programme.[10]
This study was carried out to determine the prevalent uropathogens in our area and their antibiotic sensitivity pattern to commonly used antibiotics in order to provide a database for reference. We also compared the antibiotic sensitivity pattern of the bacterial isolates between outpatients and inpatients. In the present scenario, where the antibiotic resistance pattern is changing, our study aims at outlining the recommendations for empirical treatment of UTI.
Materials and Methods
The present study was carried out in the Bacteriology Laboratory of the Department of Microbiology from July 2008 to June 2009. Urine samples were received from various outpatient Departments (OPDs) and Inpatient Departments (IPDs) of the attached tertiary care hospital. Ethical clearance not required as study was on routine laboratory isolates.
Clean catch, mid-stream urine samples were collected in sterile universal containers. Urine samples were processed within 2 h of collection and, in case of delay, the samples were refrigerated at 2-8°C for up to 6 h.
The samples were plated on Blood Agar (Himedia, Vadhani Ind. Est., LBS Marg, Mumbai, India) and MacConkey Agar media (Himedia, Vadhani Ind. Est., LBS Marg, Mumbai, India) by the semi-quantitative plating method using the calibrated loop technique (0.001 mL). Plates were incubated aerobically overnight at 37°C.
Pure growth of an isolate in a count of ≥105 colony forming units (CFU) per milliliter of urine was considered as significant bacteriuria. Growth of ≥3 isolates in a sample was considered as contamination, and a repeat sample was advised. Conventional methods of identification were used for identification of the bacterial isolates.[11]
Antimicrobial sensitivity test (AST) was done on Mueller Hinton agar (Himedia, Vadhani Ind. Est., LBS Marg, Mumbai, India) by the Kirby-Bauer technique according to the CLSI guidelines[12] using E. coli (ATCC 25922), Staphylococcus aureus (ATCC 25923) and Pseudomonas aeruginosa (ATCC 27853) as control strains.
The antibiotic discs used for the AST included:
Amikacin (30 μg), amoxycillin/clavulanic acid (20/10 μg), cefalexin (30 μg), cefixime (5 μg), cefoxitin (30 μg), cefpodoxime (10 μg), cefuroxime (30 μg), cephoperazone + sulbactam (75/10 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), gatifloxacin (5 μg), linezolid (30 μg), meropenem (10 μg), norfloxacin (10 μg), nitrofurantoin (300 μg), piperacillin (100 μg), oxacillin (1 μg), penicillin G (10 μg), tetracycline (30 μg), tobramycin (10 μg) and vancomycin (30 μg), from Himedia Laboratories (Vadhani Ind. Est., LBS Marg, Mumbai, India).
Statistical analysis
Continuous data was summarised as mean and categorical data was summarised as percentage. Chi square test was applied for analysis of categorical data. All statistical calculations were done by using by using Medcalc 14.0.0 version software (Belgium). P value < 0.05 was taken as significant for interpretation.
Results
A total of 6348 urine samples were received, of which 2753 (43.4%) were from OPD patients and 3595 (56.6%) from inpatients. Growth was present in 41.8% (2653 in 6348) samples while 55.1% (3495 in 6348) were sterile. The patients ranged from ages 1 year to 85 years, with a mean age of 46.24 years. Male and female culture positivity was 37.7% (460 of 1219) and 62.6% (759 of 1219) in OPD and 55.1% (791/1434) and 44.8% (643/1434) respectively in IPD samples.
In the OPD, culture positivity in females between ages 21 and 50 years were 322/759 (42.4%), while elderly males of 60-85 years showed a higher culture positivity rate, 191/460 (41.5%). In the IPD group, both males and females predominated in the age group 50-80 years (38.3%[303/791] and 42.3%[272/643], respectively).
Thirty-six patients presented a mixed infection with two organisms (six OPD, 30 IPD). Thus, a total of 2689 urine isolates were obtained, of which 1470 (54.7%) were IPD sample isolates and 1219 (45.3%) were OPD sample isolates. Of the 2689 isolates, 2412 (89.7%) were bacterial species and 277 (10.3%) were Candida species (spp) (81 OPD, 196 IPD).
Among the 2412 bacterial isolates, 1769 (73.4%) were Gram-negative bacilli (GNB) and 643 (26.6%) were Gram-positive cocci (GPC).
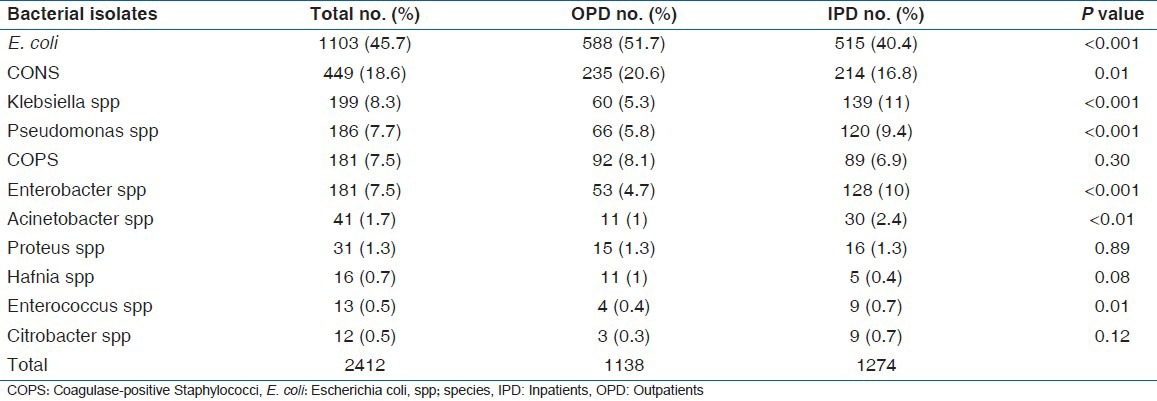
The most common bacteria isolated were E. coli (45.7%) (1103 in 2412), followed by Coagulase-negative Staphylococcus (CONS) 18.6% (449 in 2412) and Klebsiella spp 8.2% (199 in 2412) [Table 1].
Table 1.
Frequency of isolation of bacterial uropathogens list of bacterial isolates
The sensitivity pattern of GPC and GNB are shown in Tables 2 and 3, respectively.
Table 2.
Antibiotic sensitivity pattern of Gram-positive cocci
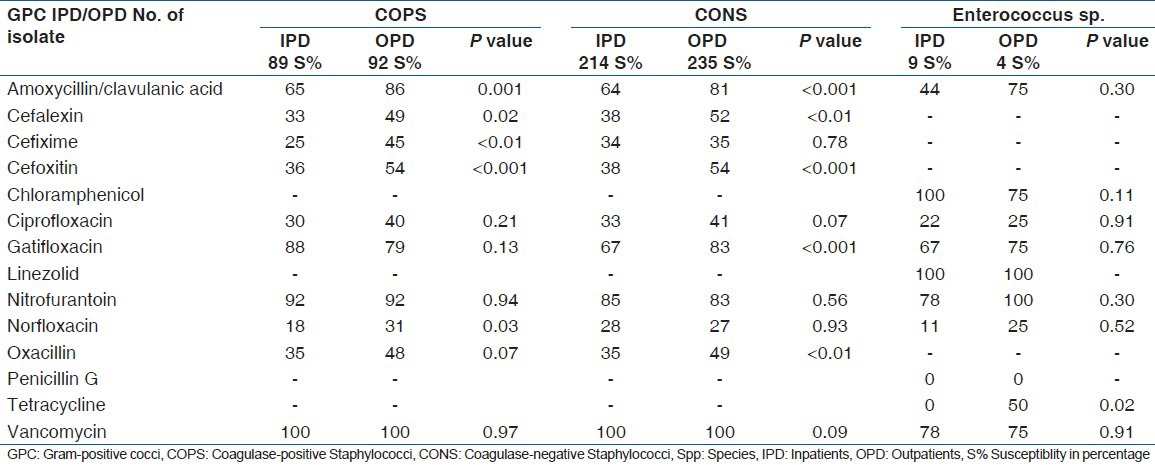
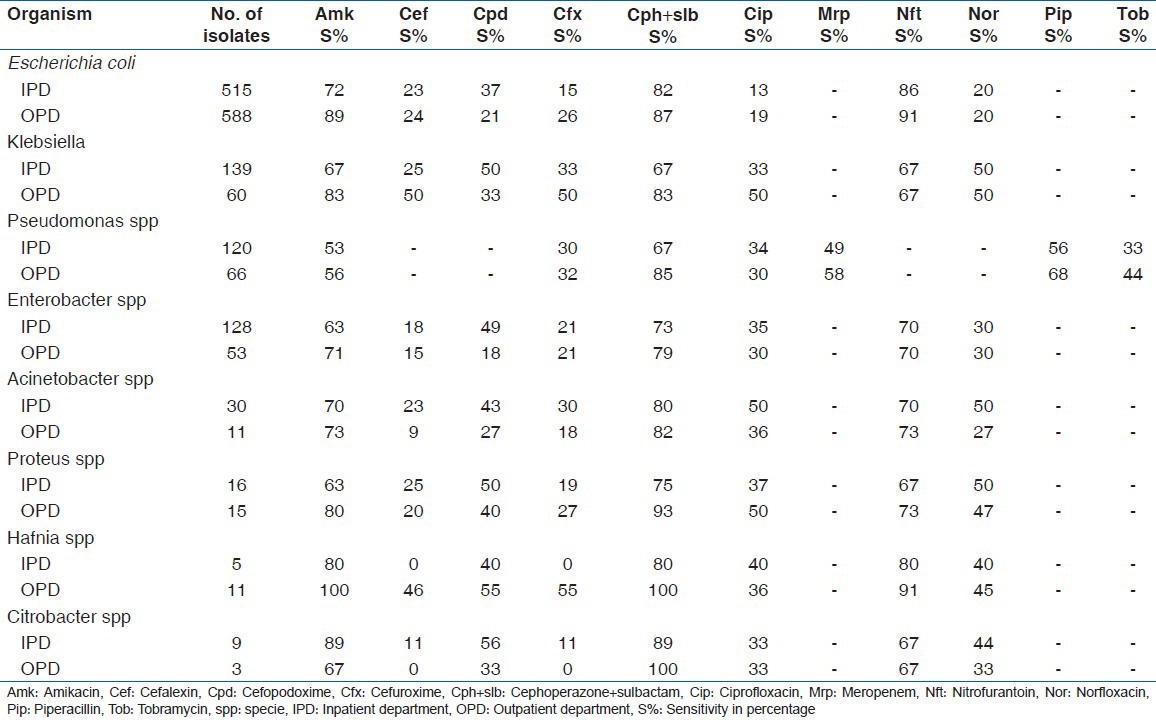
Table 3.
Susceptiblity of Gram-negative bacilli to various antibiotics
Discussion
UTI is one of the most common infections encountered, which affects all age groups including men, women and children worldwide.[4] The culture-positive rate was 41.8% (2653/6348) in the present study, and a similar culture-positive rate has been observed by other studies.[4,8,13]
The antimicrobial susceptibility patterns have changed over time, but the spectrum of agents causing UTI has remained relatively constant, with E. coli being the most common isolate.[14]
In our study, E. coli was the most common isolate (45.7%), both in the OPD and in the IPD. This is similar to studies from other tertiary care centers.[9,10,13] However, studies from some other parts of the country have shown higher isolation rates (65% to more than 90%).[15,16,17] This difference probably occurred because ours is a tertiary care center as compared with the primary and secondary care levels of these centers.
CONS, the second most common isolate in the present study, accounted for 18.6%, assupported by other studies.[14,18,19,20] Coagulase-positive Staphylococci (COPS) and Enterococcus spp were 7.5% and 0.5%, respectively. Among the Enterococcus spp, Enterococcus faecalis was 84.6% (11/13) and E. faecium accounted for 15.3% (2/13).[2,21,22]
The incidence of Enterobacter spp is high in the present study (7.5%), whereas the incidence of other enterobacteriaceae is low as compared with other studies, signifying the geographic variations prevalent in a country.[1,4,9,10,13,17] The isolation of Pseudomonas spp was 7.7% (186 of 2412), which is similar to that reported by other studies.[9,13]
We observed that E. coli, CONS and COPS isolation was higher from community patients than from hospital patients, whereas Enterobacter spp, Klebsiella spp, Pseudomonas spp and Acinetobacter spp isolation was higher from the hospital than from the community patients (Give figures and percentages to support these [Table 1]. Similar observations have been reported for E. coli, Klebsiella spp, Pseudomonas spp and Acinetobacter spp in a study from a tertiary care center in North India.[9]
Candida spp isolation was 10.3% (277/2689) in the present study, with 71% (196/277) isolates from inpatients. This is in complete agreement with another study on inpatients from Goa, which reports a similar isolation rate, thereby implying the presence of factors predisposing for fungal infections in IPD patients, like long-term antibiotic treatment, steroids, chronic illness, cancer patients or other immunocompromised conditions.[10] As ours is a retrospective study, these factors could not be correlated.
The high susceptibility of E. coli to nitrofurantoin (90.6%) and low susceptibility to norfloxacin (19.9%) and ciprofloxacin (16.3%) in the present study is in stark contrast with the study from South India by Arjunan et al., who have reported the low susceptibility to nitrofurantoin (38.8%) and relatively high susceptibility to norfloxacin (94.4%) and ciprofloxacin (77.7%), but the susceptibility reported against aminoglycosides (83.3%) is similar to the present study (80.7%).[4] This finding emphasizes the geographical variation seen in the susceptibility patterns of uropathogens to different drugs. This is important in a vast country like ours.
Fortunately, the resistance of E. coli and other Enterobacteriaceae to nitrofurantoin has remained low (range 7-34%) in our region as compared with other studies[4,17] This is probably due to the fact that nitrofurantoin has been sparingly used for UTI treatment over the past decade in the draining population of this tertiary care hospital.
The sensitivity of E.coli to amikacin and cephoperazone + sulbactam in community samples was higher than in hospital samples, and this was statistically significant for amikacin (P<0.001). This observation is also supported by other studies reporting a high amikacin resistance in inpatients.[10,13] The most effective drug against Pseudomonas spp was cephoperazone + sulbactam in both OPD and IPD samples, but the community isolates showed a higher susceptibility than hospital isolates, which was statistically significant (P < 0.05). Amikacin and cephoperazone + sulbactam are injectable drugs and, therefore, their use is more common in a hospital setup than the in community, thereby increasing the chance of development of resistant strains in the hospital surroundings. High resistance rates in nosocomial strains are typically engendered by the intense selection pressure in the hospital environment. These strains are known to be spread between patients through contaminated equipment as well as the hands of health care personnel.[23] Similarly, the AST pattern of community isolates of Hafnia spp and Enterococcus spp was different from the hospital isolates. However, the sample size of these isolates was too small to justify any attempt to correlate the findings to state the significance.
Both COPS and CONS showed more or less similar sensitivity patterns, except for nitrofurantoin, whose sensitivity was higher in COPS than in CONS (P < 0.01)
Vancomycin sensitivity was 100% in Staphylococcus sp isolates, whereas Enterococcus spp showed a 76.9% sensitivity.
Although to a different degree, all isolates showed good sensitivity to nitrofurantoin (66-93%), amikacin (53-100%) and cephoperazone + sulbactam combination (67-100%), similar to other studies.[4,10,15,17,24]
The sensitivity to fluroquinolones was low (13-50%) among all uropathogens isolated in the present study in both the OPD and the IPD samples; however, some studies have reported a high sensitivity of uropathogens to fluroquinolones.[4,15,17] Similarly, all uropathogens in the present study showed a high resistance to 1st, 2nd and 3rd generation cephalosporins (44-100%), while other studies have reported a comparatively lower resistance.[1,15,24] The high resistance to fluroquinolones and cephalosporins in the present study can be attributed to the easy access and indiscriminate use of these drugs for all types of infections, and emphasizes the role of selective drug pressure in emergence of drug-resistant mutants. It is recommended that these drugs should not be considered as first-line therapy for the empiric treatment of UTIs in this part of the country, as opposed to that recommended by other studies both from India and from abroad.[3,4,25,26] This is more so as infections caused by resistant pathogens have with them higher rates of morbidity and mortality than do infections caused by susceptible pathogens.[27]
Because a very high percentage of isolates in this study were sensitive to nitrofurantoin, this drug would be a better choice for the empiric treatment of UTI.[28,29,30] Nitrofurantoin is a narrow-spectrum antimicrobial with no systemic activity. Early formulations were associated with substantial adverse effects of the gastrointestinal system, but the current macrocrystalline formulation is well tolerated.[25,28,29] In cases with upper UTI or with systemic involvement, nitrofurantoin has a limited role, and, here, aminoglycosides or the cephoperazone + sulbactam combination would be more effective.
The increasing rate of uropathogens’ resistance to traditional empiric agents has also had an important effect on the empiric selection of antimicrobials. We recommend that constant evaluation of the antibiotic sensitivity pattern of UTI pathogens for commonly used antimicrobial agents in a particular environment should be carried out.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Tambekar DH, Dhanorkar DV, Gulhane SR, Khandelwal VK, Dudhane MN. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr J Biotechnol. 2006;5:1562–5. [Google Scholar]
- 2.Szasz M, Lehotkai N, Kristóf K, Szabó D, Nagy K. Prevalence and antimicrobial resistance of uropathogens in different inpatient wards. Acta Microbiol Immunol Hung. 2009;56:375–87. doi: 10.1556/AMicr.56.2009.4.7. [DOI] [PubMed] [Google Scholar]
- 3.Shaikh D, Ashfaq S, Shaikh K, Shaikh M, Naqvi BS, Mahmood ZA, et al. Studies on resistance/sensitivity pattern of bacteria related with urinary tract infections. Med J Islamic Wld Acad Sci. 2005;15:129–33. [Google Scholar]
- 4.Arjunan M, Al-Salamah AA, Amuthan M. Prevalence and antibiotic susceptibility of uropathogens in patients from a rural environment, Tamil Nadu. Am J Infect Dis. 2010;6:29–3. [Google Scholar]
- 5.Chamberlain NR. Urinary Tract Infections. Urethritis, Cystitis, Pyelonephritis. [Last accessed on 2012 Dec 02]. Available from: http://www.Kcom/edu/faculty/Chamberlain/website.Lectures/lecture .
- 6.Uwaezuoke JC, Ogbulie JN. Antibiotic sensitivity pattern of urinary tract pathogens in port- Harcourt, Nigeria. J Appl Sci Environ Mgt. 2006;10:103–7. [Google Scholar]
- 7.Kashef N, Djavid GE, Shahbazi S. Antimicrobial susceptibility patterns of community-acquired uropathogens in Tehran, Iran. J Infect Dev Ctries. 2010;4:202–6. doi: 10.3855/jidc.540. [DOI] [PubMed] [Google Scholar]
- 8.Aypak C, Altunsoy A, Düzgün N. Empiric antibiotic therapy in acute uncomplicated urinary tract infections and fluoroquinolone resistance: A prospective observational study. Ann Clin Microbiol Antimicrob. 2009;8:27. doi: 10.1186/1476-0711-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta V, Yadav A, Joshi RM. Antibiotic resistance patterns in uropathogens. Indian J Med Microbiol. 2002;20:96–8. [PubMed] [Google Scholar]
- 10.Kamat US, Fereirra A, Amonkar D, Motghare DD, Kulkarni MS. Epidemiology of hospital acquired urinary tract infections in a medical college hospital in Goa. Indian J Urol. 2009;25:76–80. doi: 10.4103/0970-1591.45542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baron EJ, Peterson LR, Finegold SM. 11th ed. St Louis: Mosby; 2002. Bailey and Scott's Diagnostic Microbiology; pp. 259–83. [Google Scholar]
- 12.no. 1. Vol. 27. Wayne, PA: Clinical and Laboratory Standards Institute; 2007. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 17th informational supplement, CLSI M100-S17; pp. 5–11. [Google Scholar]
- 13.Taneja N, Chatterjee SS, Singh M, Singh S, Sharma M. Pediatric urinary tract infections in a tertiary care center from North India. Indian J Med Res. 2010;131:101–5. [PubMed] [Google Scholar]
- 14.Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135:41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee B, Kulathinal S, Bhargava A, Jain Y, Kataria R. Antimicrobial resistance stratified by risk factor among Escherichia coli strains isolated from the urinary tract at a rural clinic in Central India. Indian J Med Microbiol. 2009;27:329–34. doi: 10.4103/0255-0857.55449. [DOI] [PubMed] [Google Scholar]
- 16.Kadri SM, Gash B, Rukhsana A. Antibiotic sensitivity and resistance profile of the micro-organisms responsible for urinary tract infection observed in Kashmir, India. J Indian Med Assoc. 2002;100:656,658–60. [PubMed] [Google Scholar]
- 17.Mohammed A, Mohammed S, Khan AU. Etiology and antibiotic resistance pattern of community acquired urinary tract infections in JNMC Hospital Aligarh, India. Ann Clin Microbiol Antimicrob. 2007;6:4. doi: 10.1186/1476-0711-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell RG. Urinary tract infections due to coagulase- negative staphylococci. J Clin Pathol. 1964;17:105–6. doi: 10.1136/jcp.17.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillespie WA, Sellin MA, Gill P, Stephens M, Tuckwell LA, Hilton AL. Urinary tract infection in young women, with special reference to Staphylococcus saprophyticus. J Clin Pathol. 1978;31:348–50. doi: 10.1136/jcp.31.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey RR. Significance of coagulase- negative staphylococcus in urine. J Infect Dis. 1973;127:179–81. doi: 10.1093/infdis/127.2.179. [DOI] [PubMed] [Google Scholar]
- 21.Bourdon N, Fines-Guyon M, Thiolet JM, Maugat S, Coignard B, Leclercq R, et al. Changing trends in vancomycin-resistant enterococci in French hospitals, 2001-08. J Antimicrob Chemother. 2011;66:713–21. doi: 10.1093/jac/dkq524. [DOI] [PubMed] [Google Scholar]
- 22.Marothi YA, Agnihotri H, Dubey D. Enteroccocal resistance- an overview. Indian J Med Microbiol. 2005;23:214–9. [PubMed] [Google Scholar]
- 23.Warren JW. Nosocomial Urinary Tract Infections. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 6th ed. Ch. 302. Philadelphia: Churchill Livingstone; 2005. pp. 3028–38. [Google Scholar]
- 24.Prashanth K, Badrinath S. In vitro susceptibility pattern of aceinetobacter species to commonly used cephalosporins, quinolones and aminoglycosides. Indian J Med Microbiol. 2004;22:97–103. [PubMed] [Google Scholar]
- 25.Nicolle L, Anderson PA, Conly J, Mainprize TC, Meuser J, Nickel JC, et al. Uncomplicated urinary tract infection in women. Current practice and the effect of antibiotic resistance on empiric treatment. Can Fam Physician. 2006;52:612–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Amin M, Mehdinejad M, Pourdangchi Z. Study of bacteria isolated from urinary tract infections and determination of their susceptibility to antibiotics. Jundishapur J Microbiol. 2009;2:118–23. [Google Scholar]
- 27.Holmberg SD, Solomon SL, Blake PA. Health and economic impacts of antimicrobial resistance. Rev Infect Dis. 1987;9:1065–78. doi: 10.1093/clinids/9.6.1065. [DOI] [PubMed] [Google Scholar]
- 28.Christiaens TC, De Meyere M, Verschraegen G, Peersman W, Heytens S, De Maeseneer JM. Randomized, controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. Br J Gen Pract. 2002;52:729–34. [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolle LE. Urinary tract infection: Traditional pharmacologic therapies. Am J Med. 2002;113(Suppl 1A):35–44S. doi: 10.1016/s0002-9343(02)01058-6. [DOI] [PubMed] [Google Scholar]
- 30.Hooton TM. Uncomplicated urinary tract infection. N Eng J Med. 2012;366:1028–37. doi: 10.1056/NEJMcp1104429. [DOI] [PubMed] [Google Scholar]


