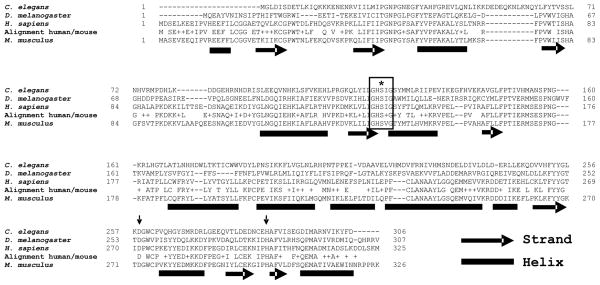
Figure 2. LDAH has conserved esterase/lipase compatible features.
LDAH is evolutionarily conserved from rice to human. The figure displays the sequences of the C. elegans, Drosophila, human, and mouse LDAH homologs. The alignment between mouse and human LDAH is shown between their sequences. A putative active site nucleophilic serine (S, marked with an asterisk) is located within a conserved typical GXSXG pentapeptide lipase catalytic motif (boxed). A conserved aspartic acid (D) and a conserved histidine (H) are pointed by arrows. The bioinformatics prediction of mLDAH secondary structure is shown under the mouse sequence.