Abstract
Although melanoma vaccines stimulate tumor antigen (TA)-specific CD8+ T cells, objective clinical responses are rarely observed. To investigate this discrepancy, we evaluated the character of vaccine-induced CD8+ T cells with regard to the inhibitory T cell co-receptors PD-1 and Tim-3 in metastatic melanoma patients who were administered tumor vaccines. The vaccines included incomplete Freund's adjuvant (IFA), CpG oligodeoxynucleotide (CpG) and the HLA-A2-restricted analog peptide NY-ESO-1 157-165V, either by itself or in combination with the pan-DR epitope NY-ESO-1 119-143. Both vaccines stimulated rapid TA-specific CD8+ T-cell responses detected ex vivo, however, TA-specific CD8+ T cells produced more IFN-γ and exhibited higher lytic function upon immunization with MHC class I and class II epitopes. Notably, the vast majority of vaccine-induced CD8+ T cells upregulated PD-1 and a minority also upregulated Tim-3. Levels of PD-1 and Tim-3 expression by vaccine-induced CD8+ T cells at the time of vaccine administration correlated inversely with their expansion in vivo. Dual blockade of PD-1 and Tim-3 enhanced the expansion and cytokine production of vaccine-induced CD8+ T cells in vitro. Collectively, our findings support the use of PD-1 and Tim-3 blockades with cancer vaccines to stimulate potent antitumor T cell responses and increase the likelihood of clinical responses in advanced melanoma patients.
Keywords: Peptide-based vaccine, PD-1, Tim-3, CD8+ T cells, Melanoma
Introduction
Although peptide-based vaccines have failed to provide significant clinical benefits in patients with advanced melanoma, the dissection of vaccine-induced T cell responses has provided the rationale for stepwise optimization of vaccine strategies (1). A major improvement of peptide vaccines with MHC class I epitopes and incomplete Freund’s adjuvant (IFA) in cancer patients has been achieved by the addition of the TLR9 ligand CpG-oligodeoxynucleotide (CpG) that stimulates strong tumor antigen (TA)-specific CD8+ T cell responses (2–4). Although the vaccine-induced CD8+ T cells are often tumor-reactive in vitro, they fail to promote tumor rejection in patients with advanced melanoma. A number of experimental studies have shown that CD4+ T cell depletion in mice with chronic infections results in major CD8+ T cell dysfunction and disease progression, suggesting that CD4+ T cell help plays a critical role in maintaining CD8+ T cell functions in the presence of high antigen load (5–7). To determine whether TA-specific CD4+ T cells augment TA-specific CD8+ T cell numbers and function in patients with advanced melanoma, two clinical trials have compared immunizations with subcutaneous (SC) injections of MHC class I epitopes versus both MHC class I and MHC class II epitopes, when emulsified in IFA (8, 9). Strikingly, IFN-γ producing TA-specific CD8+ T cells were detected less frequently in patients immunized with both class I and class II epitopes. These vaccines have not, however, included potent adjuvants to activate APCs and prime strong vaccine-induced T cells that can be detected ex vivo. Therefore, the immunological effect of CD4+ T cells in patients with advanced melanoma still needs to be thoroughly evaluated in the context of cancer vaccines with potent adjuvants.
A number of studies have shown that the inhibitory receptors PD-1 and Tim-3 are upregulated by dysfunctional TA-specific CD8+ T cells in animals and patients with advanced melanoma (10–12). Targeting PD-1 and Tim-3 with blocking antibodies enhances the expansion and function of TA-specific CD8+ T cells in vitro and in vivo, resulting in tumor rejection in experimental models. In animal models, PD-1 blockade synergizes with tumor vaccines to enhance tumor antigen-specific T cell responses and induce delayed tumor growth or partial tumor regression (13–15). In addition, vaccines appear to induce the upregulation of PD-1 expression by vaccine-induced CD8+ T cells at tumor sites (14, 15). PD-1 blockade alone represents one of the most potent therapies of advanced melanoma, inducing durable complete and partial clinical responses in a significant number of melanoma patients (16, 17). Whether PD-1 and Tim-3 are expressed by vaccine-induced TA-specific CD8+ T cells and whether they play a role in regulating the expansion and function of vaccine-induced CD8+ T cells in patients with advanced melanoma is still unknown.
In this study, we present the immunological findings from a clinical trial of immunization with IFA, CpG and the HLA-A2-restricted analog peptide NY-ESO-1 157-165V, either alone, or in combination with the pan-DR epitope NY-ESO-1 119-143, in patients with metastatic melanoma. We observed that the vast majority of vaccine-induced TA-specific CD8+ T cells detected ex vivo upregulated PD-1 and that a minority also upregulated Tim-3. The levels of PD-1 and Tim-3 expression by vaccine-induced CD8+ T cells at the time of vaccine administration inversely correlated with their expansion in vivo. In addition, we show that PD-1 and Tim-3 regulate the function and expansion of vaccine-induced CD8+ T cells in vitro.
Materials and Methods
Patients and study protocol
Twelve HLA-A2+ patients with NY-ESO-1+ stage III/IV melanoma (Supplementary Table 1) were included, after informed consent, in this phase I study approved by the University of Pittsburgh Institutional Review Board. Each vaccine was prepared as a stable emulsion composed of 2 mg CpG 7909/PF-3512676 (Pfizer Inc.), 400 μg analog peptide NY-ESO-1 157-165V alone (melanoma patient #1 [MP1] to MP5, in arm 1) or in combination with 400 μg peptide NY-ESO-1 119-143 (MP6 to MP12, in arm 2), in Montanide ISA-720 (Seppic Inc.). The final immunization volume of 4 mL was administered as four separate SC injections. Patients received 8 biweekly immunizations before clinical and immunological evaluation after 4 months of treatment. Non-progressor patients received monthly immunizations until disease progression.
Ex vivo frequency and phenotype analysis of NY-ESO-1 157-165-specific CD8+ T cells
CD8+ T lymphocytes were purified from PBMCs of patients using MACS Column Technology (Miltenyi Biotec) and incubated with PE- or APC-labeled HLA-A2/NY-ESO-1 157-165, or HLA-A2/HIVpol 476-484 tetramers (LICR, Lausanne). Next, cells were surface stained with the following antibodies: CD8-FITC or CD8-PerCP-Cy5.5, CD45RA-ECD or CD14-ECD, CD19-ECD and CD56-biotin (Beckman Coulter) with streptavidin-ECD (Invitrogen), CD4-PE-Cy7 (Beckman Coulter) or PD-1-PE-Cy7 (BioLegend) or CD28-PerCp-Cy5.5 and CCR7-PE-Cy7 (BD Pharmingen), Tim-3-PE (R&D Systems) and CD27-Alexa750 (eBioscience). A violet amine reactive dye (Invitrogen) was used to assess cell viability. In some experiments, cells were intracellularly stained with Perforin-FITC (BD Pharmingen), Granzyme A-Pacific Blue (Biolegend) and Granzyme B-APC (Invitrogen). The lower limit of detection (LLD) of these assays, calculated as the mean percentage of HIVpol 476-484 tetramer+ cells + 1.6445 x SD was estimated to be 0.001% of CD8+ T cells.
Ex vivo intracellular cytokine and Foxp3 staining
Ex vivo cytokine production assays were performed as previously reported (18). Briefly, purified CD8+ or CD4+ T cells were incubated with an equal number of non-CD3 autologous cells pulsed with either HLA-A2-restricted peptides NY-ESO-1 157-165 or HIVpol 476-484, or pan-DR peptide NY-ESO-1 119-143 or peptide HIVpol 711-725 (10 μg/mL) prior to tetramer and/or cell surface staining, followed by intracellular cytokine staining using IFN-γ-FITC, IL-2-APC, IL-4-PE (Miltenyi Biotec), TNF-Alexa700 or IL-21-PE (BD Pharmingen) antibodies. A violet amine reactive dye (Invitrogen) was used to assess the viability of the cells. Foxp3 staining was performed using a Foxp3 staining kit (eBioscience). The LLD of cytokine-producing T cells, calculated as the mean percentage of cells stained positively with isotype control antibodies for cytokine antibodies + 1.6445 x SD was 0.001% of CD4+ or CD8+ T cells.
Ex vivo CD107a degranulation assays
Purified CD8+ T cells were incubated in the presence of non-CD3 autologous cells pulsed with peptides NY-ESO-1 157-165 or HIVpol 476-484 (10 μg/mL), CD107a-FITC antibodies (BD Pharmingen), brefeldin A and Monensin (Sigma-Aldrich), prior to tetramer and cell surface staining with CD8-PerCP- Cy5.5, CD14-ECD, CD19-ECD, CD56-biotin, CD4-PE-Cy7 (Beckman Coulter) antibodies and streptavidin-ECD.
Experiments with PD-1 and Tim-3 blockade
The experiments with PD-1 and Tim-3 blockade were performed as previously described (18).
Statistics
T cell responses to the vaccines were defined as greater than a 2-fold increase in the number of tetramer+ T cells or NY-ESO-1-specific cytokine producing T cells at any time point after starting immunization as compared to pre-vaccination and as greater than 2 times the LLD. The Wilcoxon signed rank test was used to assess the significance of T cell responses. In Figs. 3 and 4, a two-sided Student’s t-test was used to compare the two arms. In Figs. 5C and 6A, a linear mixed model was used to determinate relationships between studied variables. In PD-1 and Tim-3 blockade experiments, statistical hypotheses were tested with the Wilcoxon signed rank test for paired results from the same patient. Tests were two-sided and considered significant at P < 0.05.
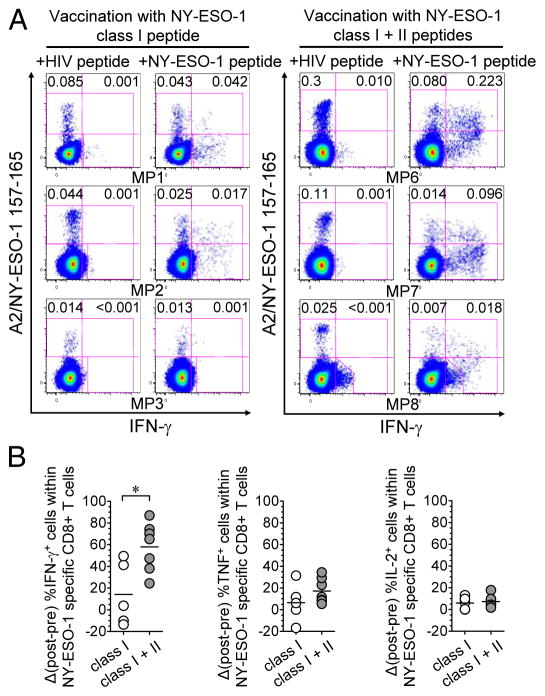
Figure 3.
Immunization with MHC class I and class II peptides, CpG and Montanide increases cytokine production by TA-specific CD8+ T cells. (A) Flow cytometry dot plots from total CD8+ T cells of three selected melanoma patients (MP) in arm 1 (left panel) and arm 2 (right panel) showing the ex vivo percentages of IFN-γ - and IFN-γ + NY-ESO-1 157-165-specific CD8+ T cells among total CD8+ T cells assessed after vaccination. (B) After- versus before- vaccination differences in the percentages of IFN-γ-, TNF- and IL-2-producing cells among total vaccine-induced NY-ESO-1-specific CD8+ T cells, in patients immunized in arm 1 (n = 5) and arm 2 (n = 7). For patients with no NY-ESO-1-specific CD8+ T cell response before vaccination, pre-vaccine percentages of cytokine-producing cells were considered to be 0. Horizontal bars, means. Open circles, arm-1 patients, grey circles, arm-2 patients. *, P < 0.05 was considered significant. Data shown are from two or more independent measurements.
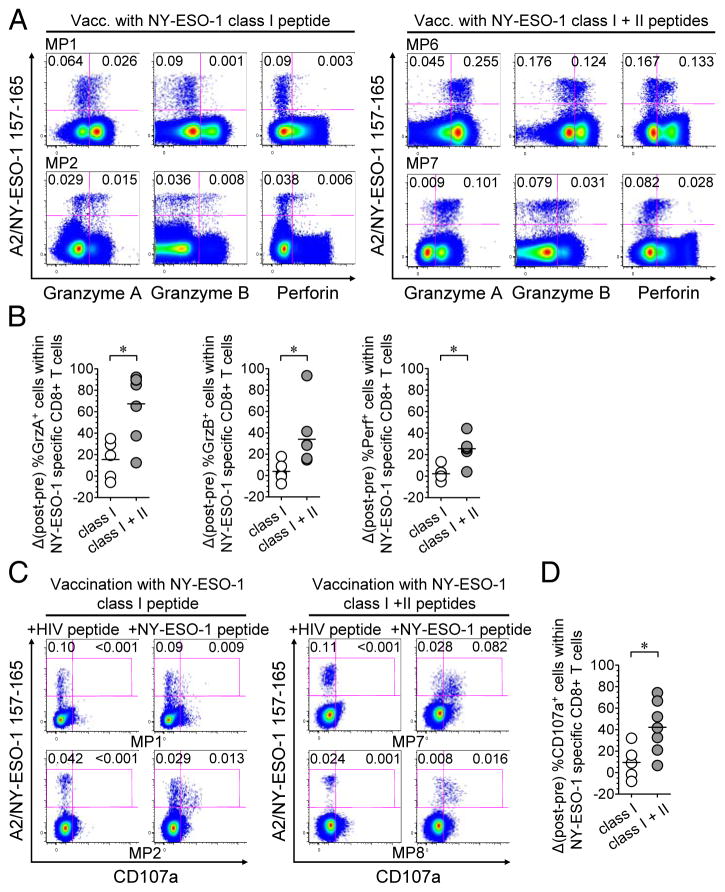
Figure 4.
Immunization with MHC class I and class II peptides augments cytotoxic potential and lytic function of TA-specific CTLs. (A) Flow cytometry dot plots of total CD8+ T cells from four selected melanoma patients (MP) in arm 1 (left panel) and arm 2 (right panel), showing ex vivo percentage of NY-ESO-1 tet+ CD8+ T cells among total CD8+ T cells that express granzyme A, granzyme B, or perforin after vaccination. (B) After- versus before- vaccination differences in the percentage of granzyme A, granzyme B and perforin-expressing cells among total vaccine-induced NY-ESO-1-specific CD8+ T cells, in patients from arm 1 (n = 5) and arm 2 (n = 7). For patients with no NY-ESO-1-specific CD8+ T cell response before vaccination, pre-vaccine percentages of cytotoxic marker-expressing cells were considered to be 0. (C) Flow cytometry dot plots of total CD8+ T cells from four melanoma patients in arm 1 (left panel) and arm 2 (right panel), showing ex vivo percentages of CD107a - and CD107a+ NY-ESO-1 tet+ CD8+ T cells among total CD8+ T cells after vaccination. (D) After- versus before- vaccination differences in the percentages of CD107a+ cells within total vaccine-induced NY-ESO-1-specific CD8+ T cells, in patients from arm 1 (n = 5) and arm 2 (n = 7). Horizontal bars, means. Open circles, arm-1 patients, grey circles, arm-2 patients. *, P < 0.05 was considered significant. Data shown are from of two independent experiments.
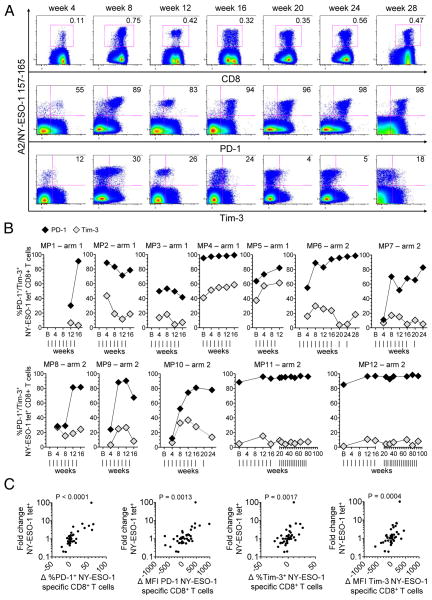
Figure 5.
Ex vivo expression of PD-1 and Tim-3 by NY-ESO-1 157-165-specific CD8+ T cells following immunizations in arms 1 and 2. (A-B) Flow cytometry dot plots from one selected melanoma patient in arm 2 (MP6) (A), and summary data for all patients vaccinated in arm 1 (n = 5) and arm 2 (n = 7) (B), showing ex vivo percentages of PD-1+ and Tim-3+ cells within total NY-ESO-1 tet+ CD8+ T cells, at different time points throughout the course of vaccination. Vertical lines, time points of vaccinations. (C) Correlation between the fold changes in NY-ESO-1 tet+ CD8+ T cell frequencies and the differences in ex vivo PD-1 and Tim-3 expression (differences in percentage and MFI of PD-1 and Tim-3 expression by NY-ESO-1 tet+ CD8+ T cells) throughout the course of vaccination. P < 0.05 was considered significant. Data shown are from two independent experiments.
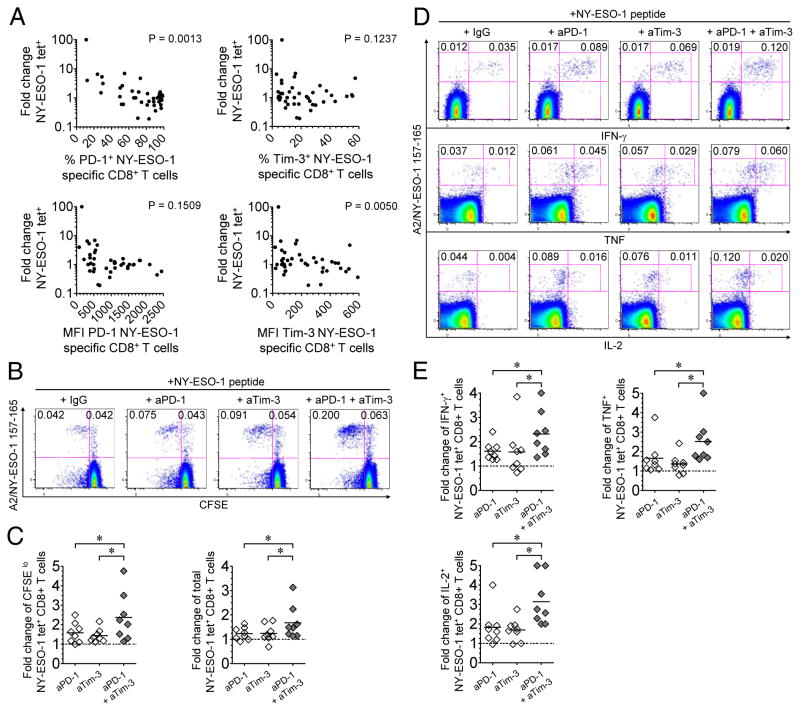
Figure 6.
The expansion of vaccine-induced TA-specific CD8+ T cells is regulated by PD-1 and Tim-3. (A) Correlation between the fold changes in NY-ESO-1 tet+ CD8+ T cell frequencies assessed between two consecutive time points throughout the course of vaccination, in both arms, and the level of PD-1 and Tim-3 expression (expressed as both percentage and MFI of expression) by NY-ESO-1-specific CD8+ T cells assessed on the first day of each corresponding interval, within each patient. P < 0.05 was considered significant. (B-E) PBMCs isolated from eight melanoma patients after 4 months of vaccination (8 immunizations) were incubated for 6 days in vitro with peptide NY-ESO-1 157-165 in the presence of blocking mAbs against PD-1 and/or Tim-3 or IgG control antibodies. Frequencies and fold changes of proliferating/CFSElo or cytokine-producing NY-ESO-1-specific CD8+ T cells were assessed after a 6-day IVS with cognate peptide and blocking antibodies, compared to IgG control antibodies. (B-C) Representative flow cytometric analysis from one melanoma patient, showing percentages of vaccine-induced CFSElo NY-ESO-1 tet+ CD8+ T cells among total CD8+ T cells (B), and fold changes in the frequencies of vaccine-induced CFSElo and total NY-ESO-1 tet+ CD8+ T cells (n = 8) (C). (D-E) Representative flow cytometric analysis from one melanoma patient, showing percentages of vaccine-induced IFN-γ-, TNF- and IL-2-producing NY-ESO-1 tet+ CD8+ T cells among total CD8+ T cells (D), and fold changes in the frequencies of vaccine-induced cytokine-producing NY-ESO-1 tet+ CD8+ T cells (n = 8) (E). *, P < 0.05 was considered significant. Horizontal bars, means. Data shown are representative of two independent experiments.
Results
Immunization with MHC class I or class I and class II peptides results in rapid and strong expansion of NY-ESO-1-specific CD8+ T cells
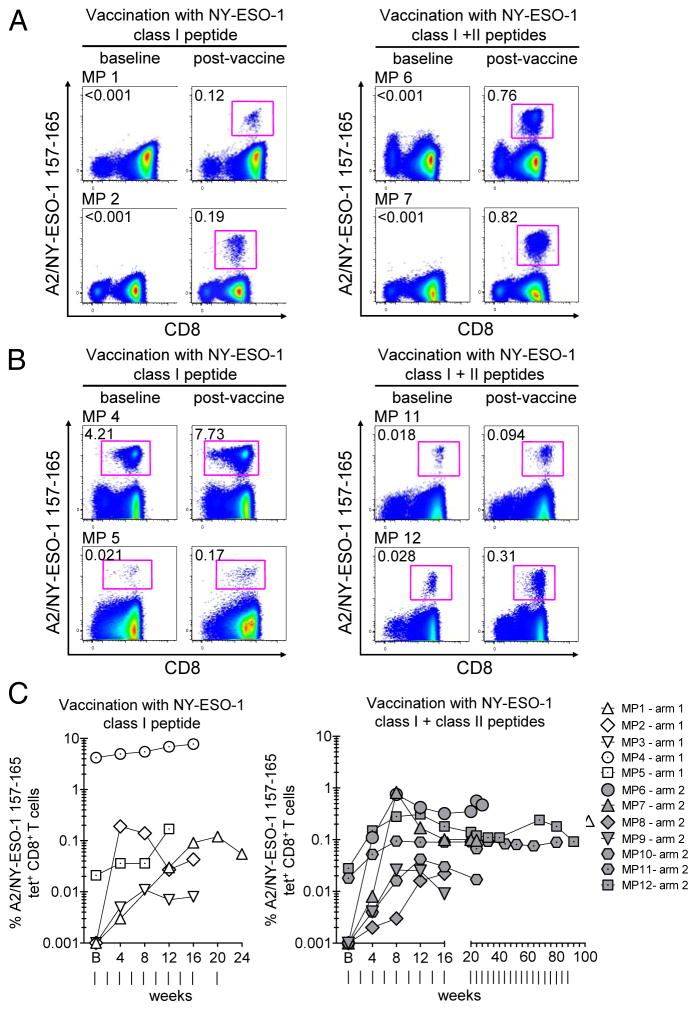
We first evaluated the ex vivo frequency of NY-ESO-1 157-165-specific CD8+ T cells (hereafter also called NY-ESO-1-specific CD8+ T cells) in PBMCs collected from melanoma patients prior to the first immunization and at different time points during the course of vaccination. Immunization with MHC class I peptide (arm 1) or MHC class I and class II peptides (arm 2) resulted in increased frequencies of NY-ESO-1-specific CD8+ T cells that were detectable with HLA-A2/NY-ESO-1 157-165 (NY-ESO-1) tetramers ex vivo in all patients after 2 immunizations (4 weeks of treatment) (Fig. 1 A-C). In patients with no detectable NY-ESO-1-specific CD8+ T cells prior to therapy, the highest frequencies of vaccine-induced NY-ESO-1-specific CD8+ T cells ranged from 0.011% to 0.19% of total CD8+ T cells in arm 1, and from 0.022% to 0.82% in arm 2 (Fig. 1A and C). In two arm-1 patients (MP4 and MP5), and two arm-2 patients (MP11 and MP12), with spontaneous NY-ESO-1-specific CD8+ T cells generated prior to the first vaccination, frequencies increased from 4.21% and 0.021% of total CD8+ T cells to 7.73% (1.8-fold increase) and 0.17% (8.1-fold increase), respectively, in arm 1, and from 0.018% and 0.028% of total CD8+ T cells to 0.094% (5.2-fold increase) and 0.31% (11.1-fold increase), respectively, in arm 2 (Fig. 1B and C). Overall, we observed a significant increase in the frequencies of NY-ESO-1-specific CD8+ T cells (P = 0.0005).
Figure 1.
Expansion of NY-ESO-1 157-165-specific CD8+ T cells following immunizations with CpG, Montanide and MHC class I peptide, alone, or in combination with MHC class II peptide. (A-B) Dot plots from total CD8+ T cells of selected melanoma patients (MP) in arm 1 (left panels) or in arm 2 (right panels) before and after vaccination. Data are the highest reached percentages after vaccination for each patient. Numbers indicate percentages of ex vivo detectable NY-ESO-1 tetramer (tet)+ CD8+ T cells among total CD8+ T cells. (C) Kinetics of NY-ESO-1 tet+ cells among total CD8+ T cells throughout the course of vaccination in all patients vaccinated in arm 1 (n = 5; left) and arm 2 (n = 7; right). Vertical lines, time points of vaccinations. Data shown are from two independent experiments.
Collectively, our findings show that peptide vaccines with CpG, IFA and MHC class I epitope alone and in combination with MHC class II epitope stimulate TA-specific CD8+ T cells that are detectable ex vivo in patients with advanced melanoma.
Immunization with both MHC class I and class II peptides stimulates Th-1-type NY-ESO-1-specific CD4+ T cells
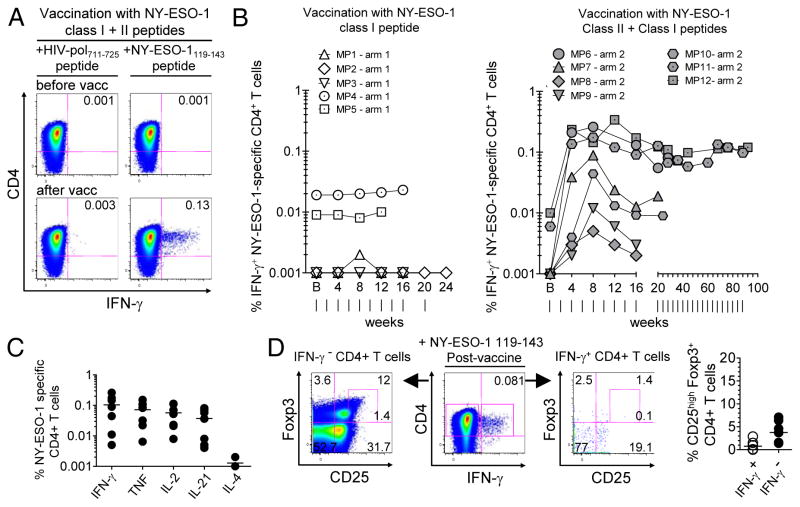
We next evaluated the frequencies of NY-ESO-1 119-143-specific CD4+ T cells (hereafter also called NY-ESO-1-specific CD4+ T cells) in PBMCs collected from melanoma patients at different time points during the course of vaccination. To this end, we assessed the frequencies of IFN-γ-producing CD4+ T cells that were detectable after ex vivo stimulation with autologous non-CD3 cells pulsed with peptide NY-ESO-1 119-143. The seven patients immunized with both MHC class I and class II peptides (arm 2) exhibited a rapid increase in the frequencies of IFN-γ-producing NY-ESO-1-specific CD4+ T cells that reached a peak after 8 to 12 weeks of vaccine therapy. In contrast, immunization with MHC class I peptide alone (arm1) had no effect on NY-ESO-1-specific CD4+ T cell expansion (Fig. 2A and B). Notably, we observed a significant increase in the frequencies of NY-ESO-1-specific CD4+ T cells in arm-2 patients (P = 0.0156), whereas no significant increase was noted in arm-1 patients (P = 0.2500). Vaccine-induced NY-ESO-1-specific CD4+ T cells displayed a Th-1 phenotype, producing IFN-γ , TNF and IL-2, but no IL-4. They also produced IL-21 (Fig. 2C and Supplementary Figure S1). We observed that the percentage of CD25highFoxp3+ cells among vaccine-induced IFN-γ-producing NY-ESO-1-specific CD4+ T cells was very low or undetectable (mean ± SD = 0.8% ± 1.1%), which is lower than that among total CD4+ T cells (3.8% ± 2.4%) (Fig. 2D), suggesting that immunization with NY-ESO-1 119-143 MHC class II peptide, CpG and IFA preferentially induced CD4+ T helper cells.
Figure 2.
Immunization with MHC class II peptide stimulates Th-1-type NY-ESO-1-specific CD4+ T cells. (A) Flow cytometry dot plots from total CD4+ T cells of one representative melanoma patient in arm 2, before and after 4 months of vaccination, showing percentages of ex vivo detectable IFN-γ-producing cells after incubation with autologous non-CD3 cells pulsed with peptide NY-ESO-1 119-143 or an irrelevant peptide (HIVpol 711-725). (B) Kinetics of ex vivo detectable IFN-γ + NY-ESO-1 119-143-specific CD4+ T cells among total CD4+ T cells throughout the course of immunization in all patients vaccinated in arm 1 (n = 5; left) and arm 2 (n = 7; right). Vertical lines, time points of vaccinations.(C) Summary data for patients in arm 2 (n = 7) showing the frequencies of cytokine-producing NY-ESO-1 119-143-specific CD4+ T cells after 2 months of vaccination. (D) Dot plots from one representative patient (left panel) and pooled data from seven patients (right panel) showing the percentages of CD25highFoxp3+ cells within ex vivo detectable IFN-γ + and IFN-γ −CD4+ T cells, after 4 months of vaccination in arm 2 and a short incubation with NY-ESO-1 119-143 peptide. Data shown are from two independent experiments.
Altogether, our data show that immunization with CpG, IFA and MHC class I and class II peptides stimulated Th-1 type NY-ESO-1-specific CD4+ T cells that were detectable ex vivo in melanoma patients.
Immunization with both MHC class I and class II peptides increases IFN-γ production, cytolytic potential and lytic capacities of TA-specific CD8+ T cells
We next assessed the capability of NY-ESO-1 157-165-specific CD8+ T cells, in PBMCs collected from melanoma patients, to produce cytokines (IFN-γ, TNF and IL-2), both before and after 3 (MP5 only) or 4 months (all other patients) of vaccination. Ex vivo frequencies of cytokine-producing NY-ESO-1-specific CD8+ T cells are presented in Fig. 3A, Supplementary Fig. S2A and Supplementary Table 2. The increase in the percentages of IFN-γ-producing cells, among total vaccine-induced NY-ESO-1-specific CD8+ T cells, was significantly higher after vaccination in arm 2 (mean ± SD = 58.1% ± 22.2%) than in arm 1 (14.2% ± 29.3%). There was no significant difference for TNF and IL-2 production between the two treatment arms (Fig. 3B).
We next assessed the intracellular expression of the cytotoxic molecules granzyme A (GrzA), granzyme B (GrzB) and perforin (Perf) by NY-ESO-1-specific CD8+ T cells present in PBMCs of patients, before and after 4 months of vaccination (3 months for MP5). Ex vivo frequencies of GrzA+, GrzB+ and Perf + NY-ESO-1-specific CD8+ T cells are presented in Fig. 4A, Supplementary Fig. S2B and Supplementary Table 3. The increase in the expression of cytotoxic markers among vaccine-induced NY-ESO-1-specific CD8+ T cells was significantly higher in patients immunized in arm 2 (mean ± SD = 67.4% ± 31.2%, 34.1% ± 28% and 25.5% ± 11.8%, for GrzA+, GrzB+ and Perf+ cells, respectively) than in patients immunized in arm 1 (15.5% ± 17.9%, 3.9% ± 9.8% and 2.3% ± 6.9%, respectively) (Fig. 4B). We also observed that the increase in the percentages of degranulating/CD107a+ cells among vaccine-induced NY-ESO-1-specific CD8+ T cells was significantly higher after vaccination in arm 2 (mean ± SD = 42.2% ± 24.3%) than after vaccination in arm 1 (9.5% ± 15.7%), which shows that immunization with both MHC class I and class II peptides enhanced lytic activity of TA-specific CD8+ T cells (Fig. 4C and D, Supplementary Fig. S2C and Supplementary Table 3).
The expansion of vaccine-induced TA-specific CD8+ T cells correlates with the upregulation of PD-1 and Tim-3 in vivo
We have previously reported that the inhibitory receptors PD-1 and Tim-3 play a critical role in regulating the expansion and functions of spontaneous NY-ESO-1-specific CD8+ T cells in vitro (12). We have also observed that PD-1 and Tim-3 upregulation by TA-specific CD8+ T cells correlates with the expression of activation markers and can be further increased upon TCR activation with cognate antigen in vitro (12, 18). To investigate whether immunization with peptides and CpG promotes the upregulation of inhibitory receptors, we next measured ex vivo expression of PD-1 and Tim-3 by vaccine-induced NY-ESO-1-specific CD8+ T cells at different time points during vaccine therapy (Fig. 5A and B). We observed that PD-1 expression was upregulated by a vast majority of NY-ESO-1-specific CD8+ T cells throughout the course of immunization in all arm-1 and arm-2 patients (mean ± SD %PD-1+ cells =73.3% ± 26%). In contrast, Tim-3 expression was increased by a minority only of cells in 10 out of 12 patients (mean ± SD %Tim-3+ cells in all patients =18.2% ± 16.3%). In all patients, a large majority of the Tim-3+ NY-ESO-1-specific CD8+ T cells co-expressed PD-1 (mean ± SD %PD-1+ cells within Tim-3+ cells = 84.4% ± 12.2%; data not shown).
We next wanted to investigate whether the expansion of NY-ESO-1-specific CD8+ T cells between immunizations correlated with changes in PD-1 and Tim-3 expression. We therefore calculated both the fold changes in vaccine-induced NY-ESO-1-specific CD8+ T cell frequencies and the differences in PD-1 and Tim-3 expression levels (differences in both percentage and mean fluorescence intensity [MFI] of PD-1 and Tim-3 expression by NY-ESO-1 tet+ CD8+ T cells) between two consecutive time points (corresponding to the two nearest and consecutive available blood draws over 1 or 2- month intervals) throughout the course of vaccination (up to 52 weeks) for each patient. We observed a positive correlation between the fold change in vaccine-induced NY-ESO-1-specific CD8+ T cell frequencies and the upregulation of PD-1 and Tim-3 (Fig. 5C), suggesting that the greater the vaccine-induced CD8+ T cell expansion following immunization, the greater the levels of PD-1 and Tim-3 expression.
The expansion of vaccine-induced TA-specific CD8+ T cells is regulated by PD-1 and Tim-3
We next investigated whether the expansion of vaccine-induced NY-ESO-1- specific CD8+ T cells between two consecutive time points (1- or 2-month intervals, up to 52 weeks of vaccination), correlates with the level of PD-1 and Tim-3 expression at the time of immunization. We observed a negative correlation between fold changes in vaccine-induced NY-ESO-1-specific CD8+ T cell frequencies in vivo and the percentage of cells expressing PD-1, or the MFI of cells expressing Tim-3 at the time of immunization (Fig. 6A). We next evaluated the effects of PD-1 and Tim-3 pathway blockade on the expansion and function of vaccine-induce TA-specific CD8+ T cells in vitro. CFSE-labeled PBMCs isolated from eight melanoma patients after 4 months of vaccination (8 immunizations) were incubated for 6 days with NY-ESO-1 157-165 peptide in the presence of blocking mAbs against PD-1 and/or Tim-3 or IgG control antibodies. The frequencies of proliferating (CFSElo) and total NY-ESO-1 tet+ CD8+ T cells increased after incubation with anti-PD-1, anti-Tim-3 or both mAbs when compared to incubation with IgG control antibodies, resulting in 1.6-, 1.4- and 2.4-fold changes in the frequencies of CFSElo NY-ESO-1 157-165-specific CD8+ T cells, respectively, and in 1.2-, 1.3- and 1.6-fold changes in the frequencies of total NY-ESO-1-specific CD8+ T cells, respectively (Fig. 6B and C and Supplementary Figure S3), showing an additive effect of PD-1 and Tim-3 blockades on vaccine-induced TA-specific CD8+ T cell expansion.
In addition, the frequencies of vaccine-induced NY-ESO-1-specific CD8+ T cells that produced cytokines increased after incubation in the presence of cognate peptide and anti-PD-1 mAbs, when compared to IgG control antibodies, resulting in 1.6-fold, 1.6-fold and 1.8-fold changes in the frequencies of IFN-γ-, TNF- and IL-2-producing NY-ESO-1-specific CD8+ T cells, respectively (Fig. 6D and E and Supplementary Figure S4). The frequencies of cytokine-producing vaccine-induced NY-ESO-1-specific CD8+ T cells further increased in the presence of both anti-PD-1 and anti-Tim-3 mAbs (Fig. 6D and E and Supplementary Figure S4).
Collectively, our findings show that the levels of PD-1 and Tim-3 expression by vaccine-induced TA-specific CD8+ T cells appear to be negatively correlated with the expansion of TA-specific CD8+ T cells in vivo following immunizations. They also show that PD-1 and Tim-3 blockades further augment the expansion and cytokine production of vaccine-induced TA-specific CD8+ T cells.
Side effects and clinical outcome
We observed no severe toxicity (Supplementary Table 4). None of the patients developed objective clinical responses. Among the five patients immunized in arm 1, one patient remained stable for 6 months, three patients progressed after 4 months and one patient progressed after 3 months. Out of seven patients immunized in arm 2, two patients progressed after 4 months while other patients remained stable for 6 months (2 patients), 7 months (one patient), 22 months (one patient) and 24 months (one patient) (Supplementary Table 4).
Discussion
In this study, we report the capability of peptide vaccines with MHC class I or both MHC class I and class II epitopes, in combination with CpG and IFA, to rapidly stimulate TA-specific CD8+ T cells, which are detected ex vivo, in patients with advanced melanoma. Notably, vaccine -induced TA-specific CD8+ T cells produced more IFN-γ and exhibited higher cytotoxic potential and lytic functions in patients immunized with both MHC class I and class II peptides when compared to MHC class I peptide alone, which supports a role for CD4 T-cell help in enhancing antitumor CTL responses in vivo. These findings are in agreement with a number of experimental studies of chronic viral infections in animal and humans. Loss of CD4 T-cell help correlates with severe CD8+ T cell dysfunction and disease progression (5, 6, 19), whereas adoptive transfer of LCMV-specific CD4+ T cells into chronically infected mice enhances the function of exhausted CD8+ T cells (20). In addition, in vivo stimulation of HIV-specific CD4+ T cells augments the lymphoproliferative functions of HIV-specific CD8+ T cells in patients with chronic infection (21).
The capability of cancer vaccines with CD4 helper epitopes to stimulate potent TA-specific CD8+ T cell responses in patients with advanced melanoma remains elusive. To the best of our knowledge, only two melanoma peptide vaccine trials have previously compared immunization with MHC class I versus both MHC class I and class II epitopes. Phan et al. immunized stage IV melanoma patients with HLA-A2-restricted peptides derived from gp100 and Melan-A/MART-1, either alone or in combination with one gp100 HLA-DR4 peptide, in IFA. They detected TA-specific CD8+ T cell responses by IFN-γ ELISPOT after in vitro sensitization assays in 18 of 19 patients immunized with MHC class I peptides alone, and in 8 of 16 patients immunized with both MHC class I peptides and the HLA-DR4 peptide (8). In a large multicenter randomized trial, Slingluff et al immunized measurable stage IV melanoma patients with 12 MHC class I peptides, alone or in combination with either a T-helper tetanus peptide, or a mixture of MHC class II peptides, in IFA plus GM-CSF(9). Strikingly, the response rates, as determined by IFN-γ ELISPOT after in vitro sensitization, were lower in patients treated with both MHC class I and II peptides, than in patients treated with MHC class I peptides, alone, or with MHC class I peptides in combination with tetanus peptide. These investigators also reported that immune responses to MHC class II epitopes were significantly associated with clinical responses and overall survival. Although, the reasons behind the poor immunogenicity of these two peptide vaccines with MHC class I and class II peptides in IFA have not been fully investigated, a likely hypothesis is the stimulation of TA-specific Tregs in the absence of potent adjuvants. We and others have previously reported that tumor antigens can spontaneously induce low frequencies of TA-specific CD4+ Tregs in patients with advanced melanoma (22–24). It is therefore possible that peptide vaccines that do not include potent adjuvants expand TA-specific Tregs. In this study, however, we show that CpG-based vaccine does not expand CD25highFoxp3+ NY-ESO-1-specific CD4+ Tregs.
One critical finding is the upregulation of the inhibitory receptors PD-1 and Tim-3 by vaccine-induced CD8 + T cells. We show that the majority of vaccine-induced CD8+ T cells upregulate PD-1, while a minority also upregulate Tim-3. PD-1 and Tim-3 upregulation correlates with the expansion of vaccine-induced CD8+ T cells following immunizations. Therefore, the inhibitory receptors PD-1 and Tim-3, which are co-expressed by tumor-induced exhausted CD8+ T cells present in patients with advanced cancer (12), are also upregulated by freshly activated TA-specific CD8+ T cells primed by cancer vaccines. The upregulation of PD-1 by vaccine-induced CD8+ T cells occurred in patients immunized with MHC class I peptide, either alone or in combination with MHC class II peptide. This suggests that while vaccine-induced TA-specific CD4+ T cells improve the functionality of vaccine-induced TA-specific CD8+ T cells, they do not impede the upregulation of PD-1 and Tim-3 by these cells. Interestingly, the longitudinal evaluation of vaccine-induced CD8+ T cells throughout the course of immunizations showed that the levels of PD-1 and Tim-3 expression by vaccine-induced TA-specific CD8+ T cells at the time of immunization inversely correlate with their expansion, suggesting that PD-1 and Tim-3 play a critical role in regulating the expansion of vaccine-induced CD8+ T cells in vivo. In support of this observation, we further show that PD-1 and Tim-3 blockades enhanced the expansion of vaccine-induced TA-specific CD8+ T cells in vitro. In addition, while vaccination with both MHC class I and class II peptides increased IFN-γ production by vaccine-induced CD8+ T cells, PD-1 and Tim-3 blockades further augmented the frequency of IFN-γ-, TNF- and IL-2-producing CD8+T cells, enhancing their overall functionality.
In summary, our data demonstrate that peptide vaccines with CpG, IFA and MHC class I and class II peptides stimulate TA-specific CTLs with enhanced IFN-γ production, cytotoxic potential and lytic capacities that upregulate PD-1 and Tim-3. They also show that PD-1 and Tim-3 regulate the expansion of vaccine-induced CD8+ T cells throughout the course of immunization and that PD-1 and Tim-3 blockades further enhance the expansion and function of vaccine-induced CD8+ T cells. Altogether, these findings strongly support the use of PD-1 and Tim-3 blockades, in combination with peptide vaccines and potent adjuvant, for robust expansion of vaccine-induced TA-specific CTLs, and increased likelihood of clinical benefits for patients with advanced melanoma. Such a therapeutic strategy could prove useful to the melanoma patients who do not respond to anti-PD-1 antibody therapy alone, possibly due to the lack of spontaneous TA-specific CD8+ T cells at tumor sites and PD-L1 expression by melanoma cells upon IFN-γ production by T cells (16, 25). Such patients may be more likely to respond favorably to the combination of PD-1 and Tim-3 blockades with cancer vaccines.
Supplementary Material
Acknowledgments
We thank Dr. Lisa Borghesi and Mr. Dewayne Falkner of the Flow Facility of the University of Pittsburgh, Department of Immunology for their technical support. We also thank Ms. Sabina Robinson for editorial assistance.
Grant Support: This work was supported by grants NIH/NCI R01CA90360, R01CA112198 and R01CA157467 (to H.M. Zarour), UL1 RR024153 and UL1TR000005 (CTSI), R01 NS045937 and P01 AI07378 9 (to V. K. Kuchroo) and ACS RSG-11-057-01-LIB (to A. C. Anderson)
Footnotes
Conflict of interest: Arthur Krieg has ownership interest (including patents) in Pfizer. Vijay K. Kuchroo is a founder of, and has a financial interest in, CoStim Pharmaceuticals, a company that is developing novel biologics targeting immune checkpoint inhibitors. Ana C. Anderson is a paid member of the Scientific Advisory Board of CoStim Pharmaceuticals. The other authors have no conflicting financial interests.
References
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 3.Fourcade J, Kudela P, Andrade Filho PA, Janjic B, Land SR, Sander C, et al. Immunization with analog peptide in combination with CpG and montanide expands tumor antigen-specific CD8+ T cells in melanoma patients. J Immunother. 2008;31:781–91. doi: 10.1097/CJI.0b013e318183af0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speiser DE, Lienard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–46. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–63. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–71. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan GQ, Touloukian CE, Yang JC, Restifo NP, Sherry RM, Hwu P, et al. Immunization of patients with metastatic melanoma using both class I- and class II- restricted peptides from melanoma-associated antigens. J Immunother. 2003;26:349–56. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slingluff CL, Jr, Lee S, Zhao F, Chianese-Bullock KA, Olson WC, Butterfield LH, et al. A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602) Clin Cancer Res. 2013;19:4228–38. doi: 10.1158/1078-0432.CCR-13-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–51. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 12.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–38. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 15.Sierro SR, Donda A, Perret R, Guillaume P, Yagita H, Levy F, et al. Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunity. Eur J Immunol. 2011;41:2217–28. doi: 10.1002/eji.201041235. [DOI] [PubMed] [Google Scholar]
- 16.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, et al. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–96. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–62. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 20.Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, et al. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci U S A. 2011;108:21182–7. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med. 2004;200:701–12. doi: 10.1084/jem.20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fourcade J, Sun Z, Kudela P, Janjic B, Kirkwood JM, El-Hafnawy T, et al. Human tumor antigen-specific helper and regulatory T cells share common epitope specificity but exhibit distinct T cell repertoire. J Immunol. 2010;184:6709–18. doi: 10.4049/jimmunol.0903612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francois V, Ottaviani S, Renkvist N, Stockis J, Schuler G, Thielemans K, et al. The CD4(+) T-cell response of melanoma patients to a MAGE-A3 peptide vaccine involves potential regulatory T cells. Cancer Res. 2009;69:4335–45. doi: 10.1158/0008-5472.CAN-08-3726. [DOI] [PubMed] [Google Scholar]
- 24.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, et al. Tumor-Specific Human CD4(+) Regulatory T Cells and Their Ligands. Implications for Immunotherapy. Immunity. 2004;20:107–18. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 25.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science translational medicine. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.