Figure 6. AGAP2 regulated β2AR recycling.
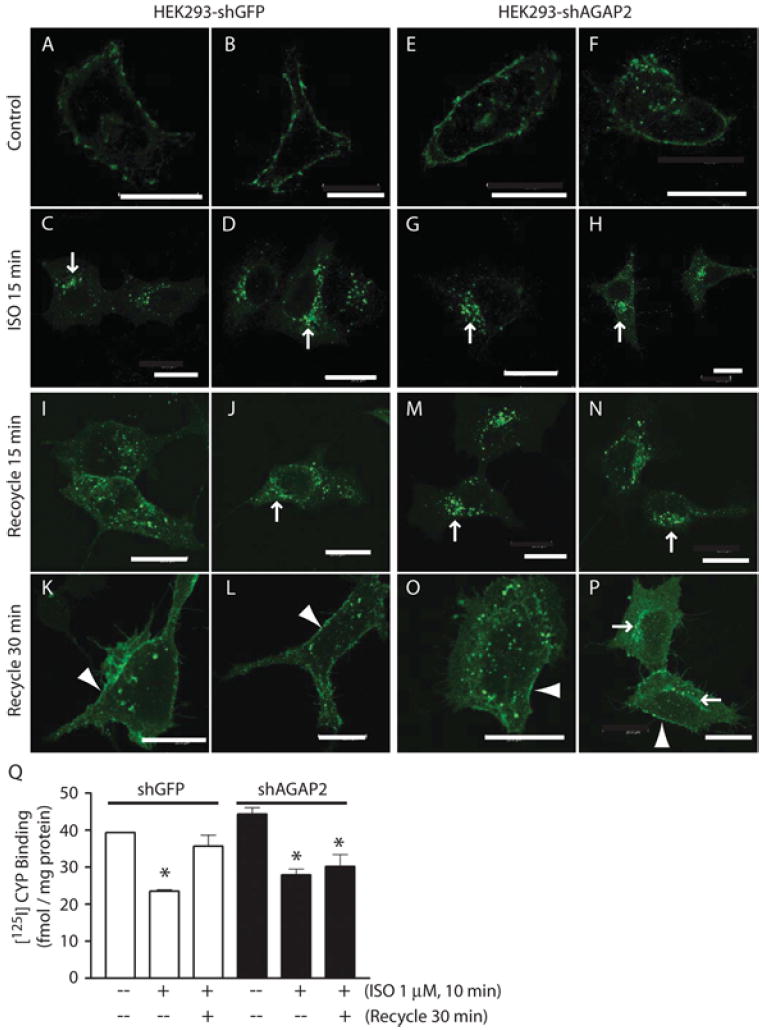
(A–D) Distribution of FLAG–β2AR in control HEK-293 cells. Cells were transfected with FLAG–β2AR cDNA for 24 h and replated on fibronectin-coated coverslips in DMEM containing 0.2% FBS for 6 h. FLAG–β2ARs, as stained using anti-FLAG antibody, were detected on the plasma membrane of resting cells (A and B) and were concentrated on the perinuclear recycling endosomes at 15 min of ISO stimulation (C and D, arrows). (E–H) Distribution of FLAG–β2AR in HEK-293 cells with stable knockdown of AGAP2. Cells were transfected with FLAG–β2AR cDNA and processed as described above. FLAG-tagged β2ARs were distributed on the plasma membrane of resting cells (E and F), and were enriched on the perinuclear recycling endosomes at 15 min of ISO stimulation (G and H, arrows). (I–L) Recycling of FLAG-β2AR in control HEK-293 cells. Cells were stimulated with ISO for 10 min, washed and incubated with DMEM containing 0.2% FBS for 15 min (I and J) or 30 min (K and L). At 15 min, FLAG-tagged β2ARs were recycling from the perinuclear region to the periphery and by 30 min they reached the plasma membrane (K and L, arrowhead). (M–P) Recycling of FLAG–β2ARs in HEK-293 cells with stable knockdown of AGAP2. At 15 min of recycling, most of the FLAG–β2AR signals were still present in the perinuclear region (M and N, arrows). By 30 min, FLAG-tagged β2ARs were present in perinuclear regions (P, arrows) or on endosomes evenly distributed throughout the cytosol (O). A small fraction of FLAG-tagged β2ARs reached plasma membrane (O and P, arrowhead). Scale bars, 20 μm. (Q) AGAP2 affected recycling of endogenous β2ARs. HEK-293 cells with or without stable AGAP2 knockdown were treated, or not, with ISO (1 μM, 10 min). Cells were washed and lysed to prepare plasma membrane, or washed and incubated with medium for an additional 30 min before plasma membrane preparation. The amount of β2ARs was determined by 125I-cyanopindolol binding and non-specific binding was determined using alprenolol. *P <0.05 compared with control.
