Table 4.
Hepatitis C Virus Direct-Acting Antiviral Structure and Likely Cost Limiting Raw Material in Production
| HCV DAA Agent | HCV DAA Structure and Retrosynthesis | Comparator HIV Agent |
|---|---|---|
| Ribavirin C8H12N4O5 MW: 244 g/mol | 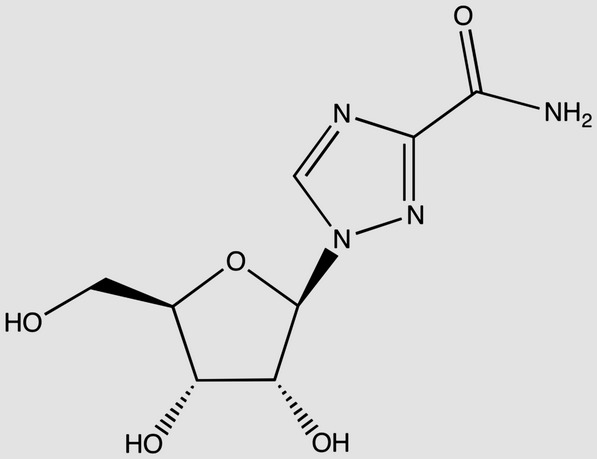 |
Zidovudine C10H13N5O4 MW: 267 g/mol |
| Daclatasvir C40H50N8O6 MW: 739 g/mol |
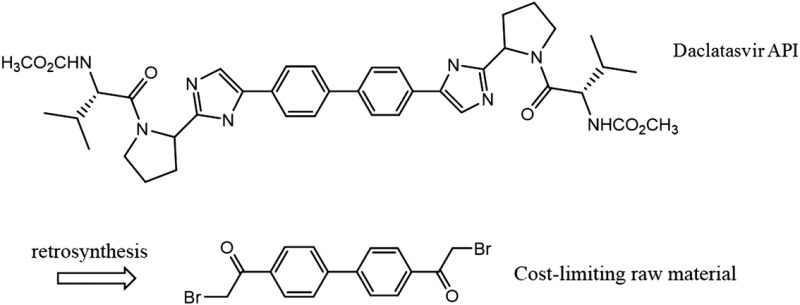 |
Atazanavir C38H52N6O7 MW: 705 g/mol |
| Sofosbuvira C22H29FN3O9P MW: 529 g/mol |
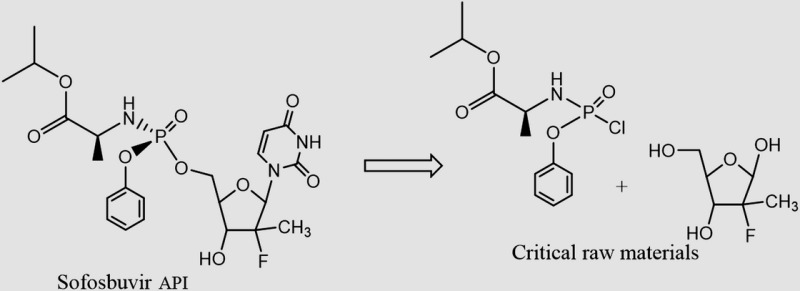 |
TDF C23H34N5O14P MW: 636 g/mol |
| Faldaprevir C40H49BrN6O9S MW: 870 g/mol |
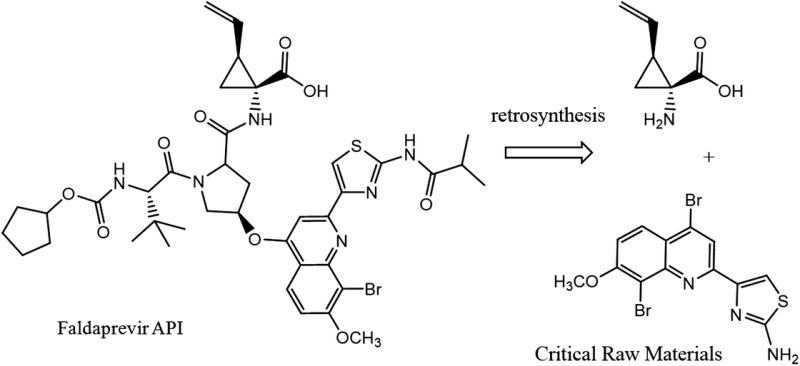 |
Darunavir C27H37N3O7S MW: 548 g/mol |
| Simeprevir C38H47N5O7S2 MW: 750 g/mol |
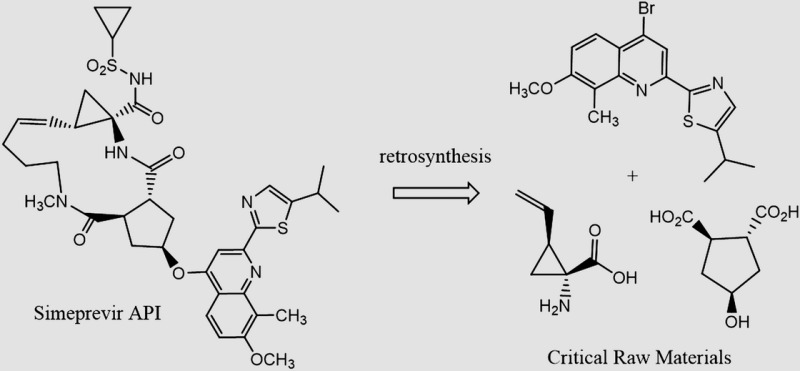 |
Lopinavir/r C37H48N4O5 MW: 629 g/mol |
Abbreviations: API, active pharmaceutical ingredients; DAA, direct-acting antiviral; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MW, molecular weight; TDF, tenofovir.
a Sofosbuvir is presumably synthesized as a mixture of diastereomers on phosphorous. Only one of these 2 diastereomers is desired in the active pharmaceutical ingredients. Separation of diastereomers can be a substantial contributor to the cost of API production, particularly when an undesired diastereomer cannot be efficiently recycled. See US patent 7 390 791 and US application 20130065856 for the tenofovir alafenamide fumarate (TAF) prodrug version of tenofovir, which contains a “ProTide” moiety identical to that present in sofosbuvir. Efficient recycling of diastereomers has been demonstrated for TAF, but we do not presume this is the case for sofosbuvir.
