Abstract
Herpes simplex virus type 2 (HSV-2) infected women have a higher prevalence of bacterial vaginosis (BV) compared to HSV-2-seronegative women. To explore the temporal association between these conditions, we evaluated the frequency of BV episodes before and after HSV-2 acquisition in a prospective study of 406 HSV-2/HIV-1-seronegative Kenyan women, of whom 164 acquired HSV-2. Incident HSV-2 was associated with increased likelihood of BV (adjusted OR, 1.28; 95% CI, 1.05–1.56; P = .01). Our findings strengthen the evidence for a causal link between genital HSV-2 infection and disruption of the vaginal microbiota.
Keywords: Bacterial vaginosis, herpes simplex virus type 2, women, Africa
Bacterial vaginosis (BV) is a polymicrobial condition characterized by depletion of hydrogen-peroxide producing vaginal lactobacilli and overgrowth of Gardnerella vaginalis and other anaerobic bacteria [1]. Although BV is the most common cause of abnormal vaginal discharge, 50%–75% of women with BV remain asymptomatic. Bacterial vaginosis is common worldwide among women of reproductive age. In the United States, the estimated prevalence of BV among women aged 14–49 is 29% [2]. Among African women, BV prevalence has been reported to be as high as 51% [3]. Bacterial vaginosis has been associated with increased risk of sexually transmitted infections (STIs) including human immunodeficiency virus type-1 (HIV-1), and with adverse reproductive health outcomes.
Herpes simplex virus type-2 (HSV-2) is a common STI worldwide and the leading cause of genital ulcer disease [4]. Most HSV-2 infections are asymptomatic, with >80% of HSV-2 seropositive individuals asymptomatically shedding virus. It is estimated that 23% of women in the United States [5] and over 50% of women in sub-Saharan Africa are infected with HSV-2 [6]. In 2003, 536 million people were infected with HSV-2 globally [4], and HSV-2 incidence was 23.6 million new cases per year. HSV-2 infection is more common in women than men. The prevalence of this chronic infection increases with age. Among high-risk groups, HSV-2 incidence can be remarkably high. For example, we reported an annual HSV-2 incidence of 23% among high-risk women in Mombasa, Kenya [7]. Like BV, HSV-2 has been found to be a significant risk factor for HIV-1 acquisition [8].
Several studies have observed associations between HSV-2 and BV. Women with BV are more likely to acquire other STIs including HSV-2 [9]. In addition, women with prevalent HSV-2 infection have a higher incidence of BV compared to HSV-2 uninfected women [10]. This observation could suggest that HSV-2 increases the risk of BV. Alternatively, women with more frequent BV may simply be more likely to acquire HSV-2. To distinguish between these 2 possibilities, we compared women's likelihood of having BV before and after HSV-2 acquisition.
MATERIALS AND METHODS
We conducted longitudinal follow-up of women participating in an open cohort study of high-risk women in Mombasa, Kenya, between February 1993 and February 2011. The eligibility criteria to join the cohort were age 18–50 years, residing in the Mombasa area, self-identifying as exchanging sex for payment in cash or in kind, and able to provide informed consent. This study was approved by the ethics review boards of Kenyatta National Hospital and the University of Washington. All participants provided written informed consent.
Clinic Procedures
At enrollment and monthly follow-up visits, a study nurse conducted a standardized face-to-face interview covering demographic data and medical and sexual history. A study physician performed a physical examination including a pelvic speculum examination. Swabs of cervical and vaginal secretions were collected for laboratory diagnosis of STIs. Blood samples were collected for HSV-2 and HIV-1 testing. Participants were provided free outpatient medical services including treatment of STIs according to Kenyan national guidelines. If indicated, syndromic management was offered during the examination visit. Participants were asked to return for test results after 7 days. At the results visit, additional treatment was provided for infections diagnosed by laboratory testing that had not been treated with syndromic management at the prior visit.
Laboratory Procedures
Serological testing for HSV-2 was performed using a type-specific HSV-2 gG based enzyme-linked immunosorbent assay (ELISA; HerpeSelect 2, Focus Diagnostics, Cypress, CA) on archived samples. An index value of <2.1 (the ratio of the optical density [OD] of the sample to the OD of the standard calibrator) was recorded as negative. Index values greater than or equal to 2.1 were considered to be positive. We selected this cutoff as likely providing the best balance of sensitivity and specificity, based on a prior study that found 2.1 to be the optimum assay cutoff in African populations similar to our own. The study demonstrated that this cutoff had 93.9% sensitivity and 90.5% specificity, against a gold standard HSV-2 Western blot. This was in comparison to the manufacturer's cutoff of >1.1, which had a sensitivity and specificity of 98.3% and 80.3%, respectively [11]. Vaginal Gram-stained slides were scored using Nugent's criteria. Scores ≥7 were classified as BV. Vaginal saline wet preparations were assessed for the presence of motile trichomonads and yeast. Culture for Neisseria gonorrhoeae was performed on modified Thayer Martin media. Cervical Gram-stained slides were examined microscopically for the presence of Gram-negative intracellular diplococci. Beginning in 2006, endocervical samples were tested for the presence of N. gonorrhoeae and Chlamydia trachomatis by transcription mediated amplification (TMA) using the Gen-Probe Aptima GC/CT Detection System (Gen-Probe, San Diego, CA). HIV-1 serostatus was determined by ELISA (Detect HIV1/2, BioChem Immunosystems, Montreal, Canada, or PT-HIV 1,2–96, Pishtaz Teb Diagnostics, Tehran, Iran). Positive tests were confirmed using a second ELISA (Recombigen, Cambridge Biotech, Worcester, MA, or Vironostika HIV-1 Uniform II AG/AB, bioMerieux, Marcy l'Etoile, France).
Data Analyses
We included all HIV-1-seronegative women in the cohort who were initially HSV-2 seronegative. For women who acquired HIV-1 during the study, we censored visits following HIV-1 infection. The primary exposure of interest was incident HSV-2 infection. Women were considered HSV-2 uninfected prior to a positive HSV-2 test and positive thereafter. The outcome was BV, dichotomized according to the presence or absence of BV (Nugent score 7–10 vs 0–6). The prevalence of BV was compared during HSV-2-seronegative vs HSV-2-seropositive follow-up visits. The outcome (BV) was measured at multiple time points on participants in our study; thus we used generalized estimating equation (GEE) modeling to allow us to assess the association between incident HSV-2 and BV while accounting for the correlation induced by having multiple observations per individual participant. We used GEE with a logit link, exchangeable correlation structure, and robust variance estimates. Results were expressed as odds ratios (OR) with 95% confidence intervals (CI). We considered known and suspected potential confounding factors including age, place of work (bar/restaurant vs nightclub or home-based/other), education level, marital status, sexual risk behaviors, STIs, and other genital tract infections (N. gonorrhoeae, C. trachomatis, Trichomonas vaginalis, Candida albicans) hormonal contraceptive use, vaginal washing, alcohol consumption, and tobacco use. We assessed the effect of potential confounding factors one at a time on BV. In the final adjusted model, we included variables that changed the regression coefficients for HSV-2 serostatus as a predictor of BV by 10% or more. We also conducted a sensitivity analysis restricting the data to the subset of women who acquired HSV-2. Analyses were performed using PASW 18.0 (PASW Inc., Chicago, IL) and STATA 11 (StataCorp, College Station, TX).
RESULTS
Between February 1993 and February 2011, 406 women who were both HIV-1 and HSV-2 seronegative contributed 809 person-years of follow-up at 5650 visits. The median duration of follow-up was 652 days (interquartile range [IQR], 147–1852). The median age of the participants at baseline was 24 years (IQR, 22–28). Most women worked in bars (N = 244, 60%), whereas the remainder worked in nightclubs (N = 145, 36%) or at other venues (N = 17, 4%). One hundred and nineteen women (29%) reported unprotected intercourse at baseline. Bacterial vaginosis was present at baseline in 116 (29%) of participants. Ninety-two percent of the women reported that they performed vaginal washing.
There were 164 incident HSV-2 infections (incidence rate 21/100 person-years). The prevalence of BV was higher during visits after HSV-2 seroconversion compared to visits before HSV-2 seroconversion (Figure 1). After adjustment for age, incident HSV-2 infection was associated with 1.28-fold increase in the odds of BV (95% CI, 1.05–1.56; P = .01) (Table 1). The magnitude of this association was similar in sensitivity analyses limited to the 164 women who acquired HSV-2 (adjusted OR, 1.25; 95% CI, 1.00–1.57; P = .05).
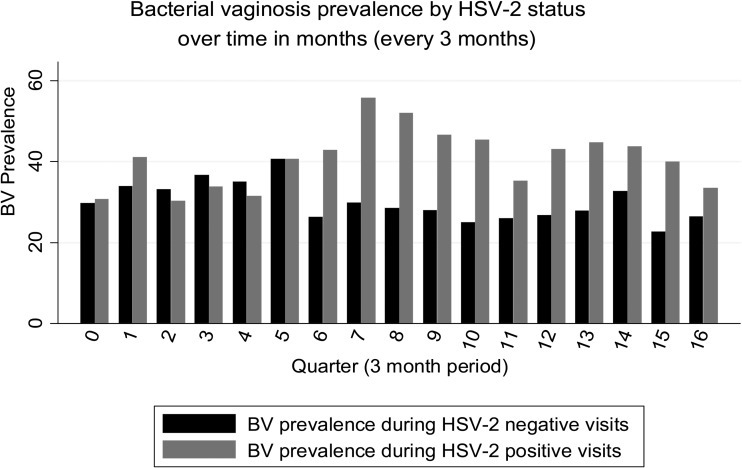
Figure 1.
Time in months since enrollment into the cohort. All women were HSV-2 negative at baseline. As the number of months in follow-up increases, there is an increase in the proportion of women with HSV-2. The proportion of women in follow-up who were HSV-2 positive at 6, 12, 18, 24, 30, 36, 42, and 48 months is 19%, 23%, 23%, 29%, 33%, 38%, 47%, and 66%, respectively. The prevalence of BV in HSV-2 negative and HSV-2 positive women is shown for every 3 months. Data are collapsed after month 48 due to sparse data after 4 years. Abbreviations: BV, bacterial vaginosis; HSV-2, herpes simplex virus type 2.
Table 1.
Prevalence of BV During HSV-2 Negative vs HSV-2 Positive Follow-up
| HSV-2 Negative Follow-up Visits | HSV-2 Positive Follow-up Visits | OR (95% CI) | P Value* | aOR** (95% CI) | P Value* | |
|---|---|---|---|---|---|---|
| N = 3769 | N = 1881 | |||||
| BV prevalence, all womena | 1173 (31.1%) | 689 (36.6%) | 1.19 (.99–1.44) | .07 | 1.28 (1.05–1.56) | .01 |
| N = 1296 | N = 1881 | |||||
| BV prevalence, HSV-2 seroconverting womenb | 424 (32.7%) | 689 (36.6%) | 1.12 (.91–1.36) | .29 | 1.25 (1.00–1.57) | .05 |
Abbreviations: aOR, adjusted odds ratio; BV,bacterial vaginosis; CI, confidence interval; HSV-2, herpes simplex virus type 2; OR, odds ratio.
a Nugent score 7–10 (vs 0–6).
b Nugent score 7–10 (vs 0–6), limited to the 164 women who acquired HSV-2.
*P-values generated from models using generalized estimating equations with a logit link, exchangeable correlation structure and robust errors.
**Model adjusted for age. Additional covariates considered for the multivariate model included place of work, education level, marital status, sexual risk behaviors, sexually transmitted infections, hormonal contraceptive use, vaginal washing, alcohol consumption, and tobacco use. However, these covariates did not confound the association between incident HSV-2 infection and BV prevalence, so were not retained in the final model.
DISCUSSION
In this cohort of HIV-1-seronegative women, we found that incident HSV-2 infection was associated with an approximately 30% increase in the odds of episodes of BV. These findings advance our understanding of the association between HSV-2 infection and the vaginal microbiota, highlighting the temporal relationship between incident HSV-2 infection and a subsequent increase in the frequency of BV. By characterizing the temporal relationship between HSV-2 acquisition and increased episodes of BV, this study makes a valuable contribution that extends beyond earlier prospective studies [10, 12]. The magnitude of the association between HSV-2 infection and increased risk of BV that was observed in this study was relatively similar to that observed in prior studies relating prevalent HSV-2 infection to BV [10, 12].
The biological mechanisms that might be responsible for increases in BV following HSV-2 infection are not clear. One possible mechanism is that intermittent HSV-2 reactivation may lead to immune activation in the genital mucosa, altering the vaginal microbiota [13]. Another plausible biological mechanism is that G. vaginalis depends on having a source of iron to thrive [14]. This may be particularly important between menses, when availability of iron could be a limiting factor. More consistent availability of iron may create an environment that facilitates the growth of G. vaginalis. Additional studies will be required to elucidate the biological link between HSV-2 infection and BV.
It is interesting to note that the increased likelihood of BV following HSV-2 infection could serve as a mechanism for enhancing further herpes transmission because BV increases genital shedding of HSV-2 [9, 12, 15]. In addition, both HSV-2 and BV have been associated with a greater risk of acquiring and transmitting HIV-1 [8]. Thus, understanding the synergistic interactions between BV and HSV-2 could have important HIV-1 prevention implications. Immunodeficiency caused by HIV-1 infection also increases the frequency and severity of HSV-2 reactivations, which could result in increased BV episodes in HIV-1-positive women. Thus, HIV-1 status is an important consideration when assessing the association between BV and HSV-2 infection.
Our study had several strengths. First, these data were prospectively collected from a large population, allowing us to accrue a substantial number of incident cases of HSV-2 infection. The large sample and prolonged follow-up provided statistical power, which allowed us to establish the temporal relationship between HSV-2 infection and increased detection of BV. Second, we had a relatively homogenous population, such that women who acquired HSV-2 were similar to those who did not. Moreover, our analyses provided similar results even when we restricted only to those women who acquired HSV-2. Third, frequent cohort visits allowed us to identify the timing of HSV-2 infection with a high level of precision.
Our results should be interpreted in the context of a number of limitations. First, this was an observational study. Thus, it is not possible to definitively prove that HSV-2 infection caused an increase in BV episodes. Second, of the 406 participants in the study, 35 (8.6%) had an initial index value between 1.1 (manufacturer's recommended cutoff) and 2.1, and then progressed to an index value >2.1. Unfortunately, we do not have Western blot data for these samples. Thus, it is possible that the cutoff of 2.1 resulted in some participants with index values between 1.1 and 2.1 being falsely classified as negative. Third, we did not collect monthly specimens for HSV-2 detection. This would have served to strengthen our argument that increases in BV may result from intermittent HSV-2 reactivation. Future studies assessing the association between HSV-2 and vaginal microbiota should consider measuring HSV-2 shedding at the time of BV assessment, and more frequently if feasible. Finally, our study population was composed of high-risk women who reported exchanging sex for payment in cash or in kind. These women's sexual risk behavior is expected to be different from the general population, and this could limit the generalizability of our findings.
By demonstrating the temporal sequence of HSV-2 infection followed by an increase in the likelihood of BV, these results strengthen the evidence for a causal link between genital herpes infection and disruption of the vaginal microbiota. Additional studies are needed to improve our understanding of the biological basis of increased BV prevalence among women who become infected with HSV-2. It will also be important to determine whether prevention or suppression of HSV-2 infection is associated with less frequent episodes of BV.
Notes
Acknowledgments. The authors wish to thank the women who participated in this study for their time and commitment to this research. We also acknowledge the contributions of our clinical, laboratory and administrative staff. We are grateful to the Municipal Council of Mombasa for providing clinical space and to Coast Provincial General Hospital for providing laboratory space.
Authors' contributions. R. S. M., L. M., J. M. B., B. A. R., G. J. -S., E. B., W. J., and J. K. conceived the question and designed the study. R. S. M. obtained funding for the study. L. M., R. S. M., J. M. B., B. A. R., and J. S. participated in collection and interpretation of the data. L. M., J. M. B., and B. A. R. conducted the data analyses. All authors participated in preparation of the article and approved the final draft for submission.
Financial support. This work was supported by the National Institute of Child Health and Human Development of the National Institutes of Health (NIH) under grant P01 HD 64915. One of the authors received training support from the Fogarty International Center (NIH 5D43-TW000007 to L. M.). Infrastructure and logistical support for the Mombasa Field Site was received from the University of Washington and Fred Hutchinson Cancer Research Center's Center for AIDS Research (grant P30-AI-27757). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 2.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109:114–20. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 3.Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis. 2005;192:1372–80. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 4.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008;86:805–12. doi: 10.2471/BLT.07.046128. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Herpes simplex virus type 2: programmatic and research priorities in developing countries. Geneva: WHO/UNAIDS. http://www.who.int/hiv/pub/sti/en/hiv_aids_2001.05.pdf . Accessed 18 Feb 2013. [Google Scholar]
- 7.Chohan V, Baeten JM, Benki S, et al. A prospective study of risk factors for herpes simplex virus type 2 acquisition among high-risk HIV-1 seronegative women in Kenya. Sex Transm Infect. 2009;85:489–92. doi: 10.1136/sti.2009.036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Wijgert JH, Morrison CS, Brown J, et al. Disentangling contributions of reproductive tract infections to HIV acquisition in African Women. Sex Transm Dis. 2009;36:357–64. doi: 10.1097/OLQ.0b013e3181a4f695. [DOI] [PubMed] [Google Scholar]
- 9.Cherpes TL, Meyn LA, Krohn MA, Hillier SL. Risk factors for infection with herpes simplex virus type 2: role of smoking, douching, uncircumcised males, and vaginal flora. Sex Transm Dis. 2003;30:405–10. doi: 10.1097/00007435-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Kaul R, Nagelkerke NJ, Kimani J, et al. Prevalent herpes simplex virus type 2 infection is associated with altered vaginal flora and an increased susceptibility to multiple sexually transmitted infections. J Infect Dis. 2007;196:1692–7. doi: 10.1086/522006. [DOI] [PubMed] [Google Scholar]
- 11.Mujugira A, Morrow RA, Celum C, et al. Performance of the Focus HerpeSelect-2 enzyme immunoassay for the detection of herpes simplex virus type 2 antibodies in seven African countries. Sex Transm Infect. 2011;87:238–41. doi: 10.1136/sti.2010.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2008;35:78–83. doi: 10.1097/OLQ.0b013e318156a5d0. [DOI] [PubMed] [Google Scholar]
- 13.Van de Perre P, Segondy M, Foulongne V, et al. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect Dis. 2008;8:490–7. doi: 10.1016/S1473-3099(08)70181-6. [DOI] [PubMed] [Google Scholar]
- 14.Piot P, Van Dyck E, Totten PA, Holmes KK. Identification of Gardnerella (Haemophilus) vaginalis. J Clin Microbiol. 1982;15:19–24. doi: 10.1128/jcm.15.1.19-24.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Infect Dis. 2005;40:1422–8. doi: 10.1086/429622. [DOI] [PubMed] [Google Scholar]