Abstract
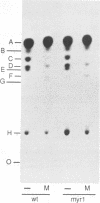
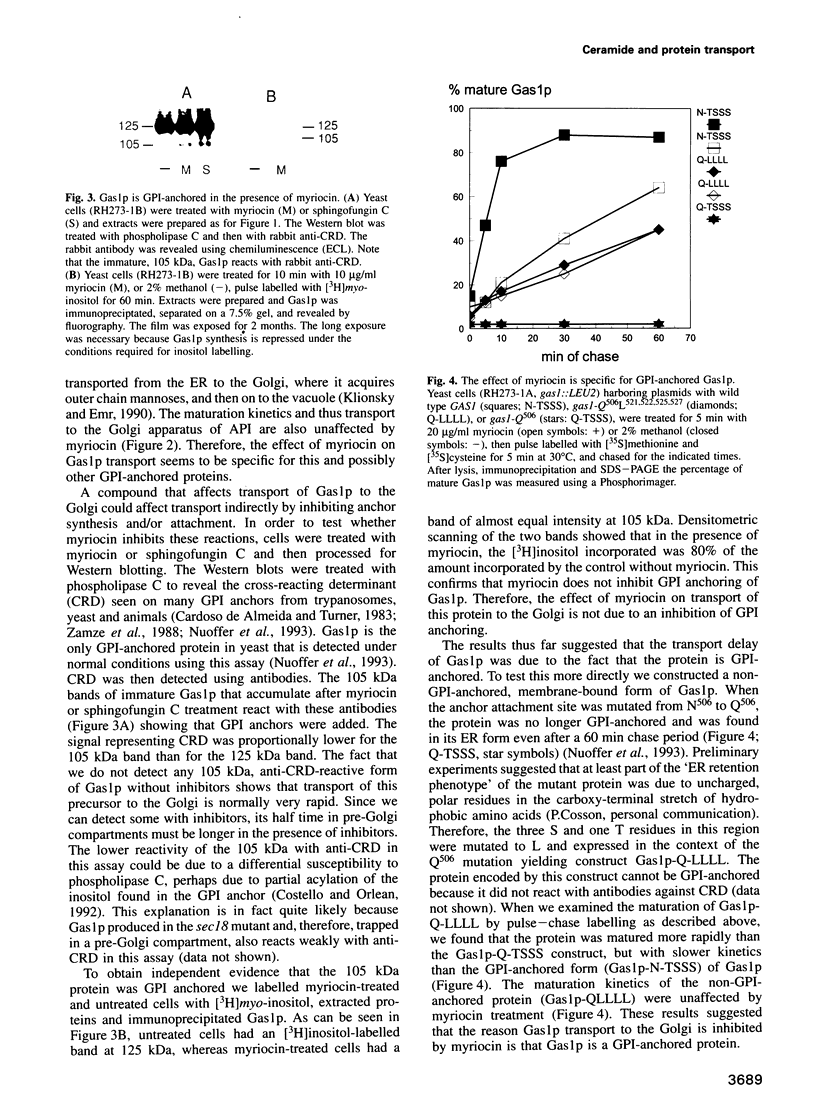
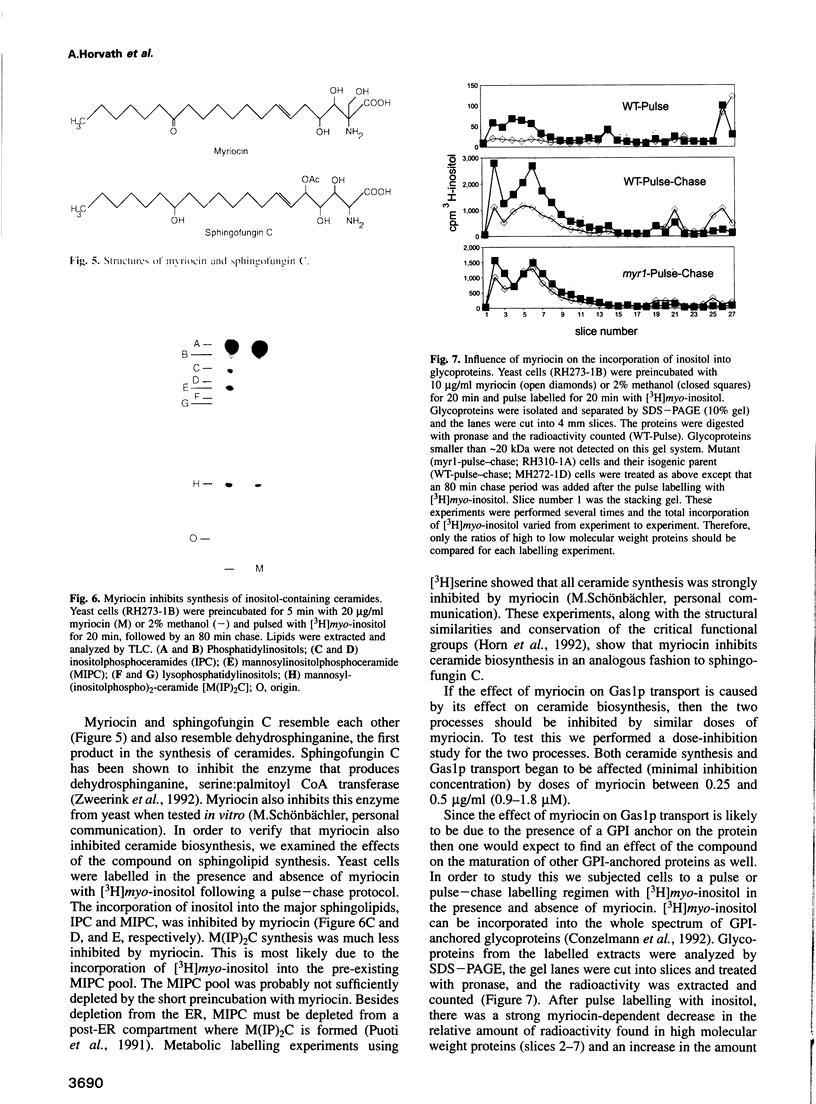
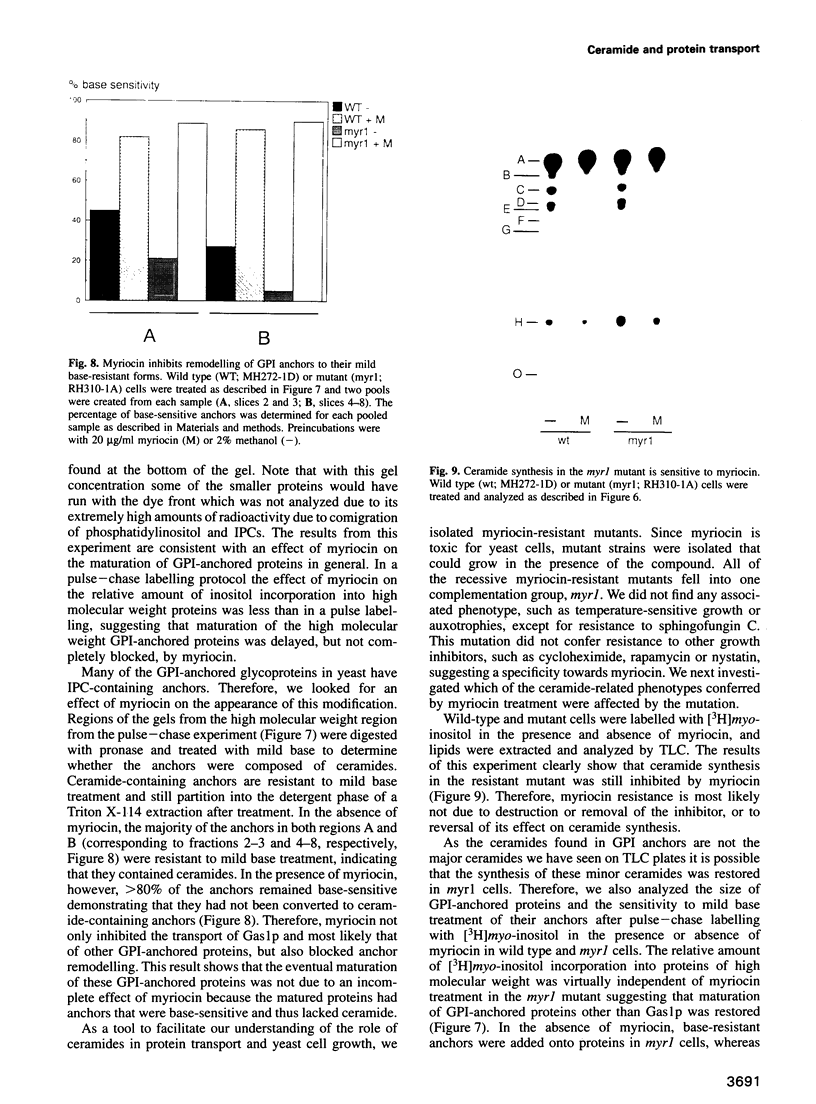
Inhibition of ceramide synthesis by a fungal metabolite, myriocin, leads to a rapid and specific reduction in the rate of transport of glycosylphosphatidylinositol (GPI)-anchored proteins to the Golgi apparatus without affecting transport of soluble or transmembrane proteins. Inhibition of ceramide biosynthesis also quickly blocks remodelling of GPI anchors to their ceramide-containing, mild base-resistant forms. These results suggest that the pool of ceramide is rapidly depleted from early points of the secretory pathway and that its presence at these locations enhances transport of GPI-anchored proteins specifically. A mutant that is resistant to myriocin reverses its effect on GPI-anchored protein transport without reversing its effects on ceramide synthesis and remodelling. Two hypotheses are proposed to explain the role of ceramide in the transport of GPI-anchored proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. A., Crise B., Rose J. K. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989 Sep 29;245(4925):1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Brown D. A. Interactions between GPI-anchored proteins and membrane lipids. Trends Cell Biol. 1992 Nov;2(11):338–343. [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Cardoso de Almeida M. L., Turner M. J. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature. 1983 Mar 24;302(5906):349–352. doi: 10.1038/302349a0. [DOI] [PubMed] [Google Scholar]
- Conzelmann A., Fankhauser C., Desponds C. Myoinositol gets incorporated into numerous membrane glycoproteins of Saccharomyces cerevisiae; incorporation is dependent on phosphomannomutase (sec53). EMBO J. 1990 Mar;9(3):653–661. doi: 10.1002/j.1460-2075.1990.tb08157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Puoti A., Lester R. L., Desponds C. Two different types of lipid moieties are present in glycophosphoinositol-anchored membrane proteins of Saccharomyces cerevisiae. EMBO J. 1992 Feb;11(2):457–466. doi: 10.1002/j.1460-2075.1992.tb05075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Riezman H., Desponds C., Bron C. A major 125-kd membrane glycoprotein of Saccharomyces cerevisiae is attached to the lipid bilayer through an inositol-containing phospholipid. EMBO J. 1988 Jul;7(7):2233–2240. doi: 10.1002/j.1460-2075.1988.tb03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello L. C., Orlean P. Inositol acylation of a potential glycosyl phosphoinositol anchor precursor from yeast requires acyl coenzyme A. J Biol Chem. 1992 Apr 25;267(12):8599–8603. [PubMed] [Google Scholar]
- Dickson R. C., Wells G. B., Schmidt A., Lester R. L. Isolation of mutant Saccharomyces cerevisiae strains that survive without sphingolipids. Mol Cell Biol. 1990 May;10(5):2176–2181. doi: 10.1128/mcb.10.5.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic V., Egerton M., Elguindi I., Raths S., Singer B., Riezman H. Yeast endocytosis assays. Methods Enzymol. 1991;194:697–710. doi: 10.1016/0076-6879(91)94051-d. [DOI] [PubMed] [Google Scholar]
- Fankhauser C., Conzelmann A. Purification, biosynthesis and cellular localization of a major 125-kDa glycophosphatidylinositol-anchored membrane glycoprotein of Saccharomyces cerevisiae. Eur J Biochem. 1991 Jan 30;195(2):439–448. doi: 10.1111/j.1432-1033.1991.tb15723.x. [DOI] [PubMed] [Google Scholar]
- Fankhauser C., Homans S. W., Thomas-Oates J. E., McConville M. J., Desponds C., Conzelmann A., Ferguson M. A. Structures of glycosylphosphatidylinositol membrane anchors from Saccharomyces cerevisiae. J Biol Chem. 1993 Dec 15;268(35):26365–26374. [PubMed] [Google Scholar]
- Fiedler K., Kobayashi T., Kurzchalia T. V., Simons K. Glycosphingolipid-enriched, detergent-insoluble complexes in protein sorting in epithelial cells. Biochemistry. 1993 Jun 29;32(25):6365–6373. doi: 10.1021/bi00076a009. [DOI] [PubMed] [Google Scholar]
- Horn W. S., Smith J. L., Bills G. F., Raghoobar S. L., Helms G. L., Kurtz M. B., Marrinan J. A., Frommer B. R., Thornton R. A., Mandala S. M. Sphingofungins E and F: novel serinepalmitoyl transferase inhibitors from Paecilomyces variotii. J Antibiot (Tokyo) 1992 Oct;45(10):1692–1696. doi: 10.7164/antibiotics.45.1692. [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990 May 18;61(4):723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Emr S. D. A new class of lysosomal/vacuolar protein sorting signals. J Biol Chem. 1990 Apr 5;265(10):5349–5352. [PubMed] [Google Scholar]
- Kluepfel D., Bagli J., Baker H., Charest M. P., Kudelski A. Myriocin, a new antifungal antibiotic from Myriococcum albomyces. J Antibiot (Tokyo) 1972 Feb;25(2):109–115. doi: 10.7164/antibiotics.25.109. [DOI] [PubMed] [Google Scholar]
- Kodukula K., Gerber L. D., Amthauer R., Brink L., Udenfriend S. Biosynthesis of glycosylphosphatidylinositol (GPI)-anchored membrane proteins in intact cells: specific amino acid requirements adjacent to the site of cleavage and GPI attachment. J Cell Biol. 1993 Feb;120(3):657–664. doi: 10.1083/jcb.120.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lester R. L., Dickson R. C. Sphingolipids with inositolphosphate-containing head groups. Adv Lipid Res. 1993;26:253–274. [PubMed] [Google Scholar]
- Lisanti M. P., Caras I. W., Rodriguez-Boulan E. Fusion proteins containing a minimal GPI-attachment signal are apically expressed in transfected MDCK cells. J Cell Sci. 1991 Jul;99(Pt 3):637–640. doi: 10.1242/jcs.99.3.637. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Ferguson M. A. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993 Sep 1;294(Pt 2):305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P., Caras I. W. Fusion of sequence elements from non-anchored proteins to generate a fully functional signal for glycophosphatidylinositol membrane anchor attachment. J Cell Biol. 1991 Dec;115(6):1595–1600. doi: 10.1083/jcb.115.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuoffer C., Horvath A., Riezman H. Analysis of the sequence requirements for glycosylphosphatidylinositol anchoring of Saccharomyces cerevisiae Gas1 protein. J Biol Chem. 1993 May 15;268(14):10558–10563. [PubMed] [Google Scholar]
- Nuoffer C., Jenö P., Conzelmann A., Riezman H. Determinants for glycophospholipid anchoring of the Saccharomyces cerevisiae GAS1 protein to the plasma membrane. Mol Cell Biol. 1991 Jan;11(1):27–37. doi: 10.1128/mcb.11.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. L., Lester R. L. The phosphoinositol sphingolipids of Saccharomyces cerevisiae are highly localized in the plasma membrane. J Bacteriol. 1991 May;173(10):3101–3108. doi: 10.1128/jb.173.10.3101-3108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto W. J., Wells G. W., Lester R. L. Characterization of enzymatic synthesis of sphingolipid long-chain bases in Saccharomyces cerevisiae: mutant strains exhibiting long-chain-base auxotrophy are deficient in serine palmitoyltransferase activity. J Bacteriol. 1992 Apr;174(8):2575–2581. doi: 10.1128/jb.174.8.2575-2581.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puoti A., Desponds C., Conzelmann A. Biosynthesis of mannosylinositolphosphoceramide in Saccharomyces cerevisiae is dependent on genes controlling the flow of secretory vesicles from the endoplasmic reticulum to the Golgi. J Cell Biol. 1991 May;113(3):515–525. doi: 10.1083/jcb.113.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald A. G., Machamer C. E., Pagano R. E. Effects of a sphingolipid synthesis inhibitor on membrane transport through the secretory pathway. Biochemistry. 1992 Apr 14;31(14):3581–3590. doi: 10.1021/bi00129a005. [DOI] [PubMed] [Google Scholar]
- Rutledge T., Cosson P., Manolios N., Bonifacino J. S., Klausner R. D. Transmembrane helical interactions: zeta chain dimerization and functional association with the T cell antigen receptor. EMBO J. 1992 Sep;11(9):3245–3254. doi: 10.1002/j.1460-2075.1992.tb05402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasek V., Sailer M., Vokoun J., Musílek V. Production of thermozymocidin (myriocin) by the pyrenomycete Melanconis flavovirens. J Basic Microbiol. 1989;29(6):383–390. doi: 10.1002/jobm.3620290619. [DOI] [PubMed] [Google Scholar]
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Smith S. W., Lester R. L. Inositol phosphorylceramide, a novel substance and the chief member of a major group of yeast sphingolipids containing a single inositol phosphate. J Biol Chem. 1974 Jun 10;249(11):3395–3405. [PubMed] [Google Scholar]
- Young W. W., Jr Molecular approaches to studying the intracellular trafficking of glycosphingolipids. Adv Lipid Res. 1993;26:161–179. [PubMed] [Google Scholar]
- Zamze S. E., Ferguson M. A., Collins R., Dwek R. A., Rademacher T. W. Characterization of the cross-reacting determinant (CRD) of the glycosyl-phosphatidylinositol membrane anchor of Trypanosoma brucei variant surface glycoprotein. Eur J Biochem. 1988 Oct 1;176(3):527–534. doi: 10.1111/j.1432-1033.1988.tb14310.x. [DOI] [PubMed] [Google Scholar]
- Zurzolo C., Lisanti M. P., Caras I. W., Nitsch L., Rodriguez-Boulan E. Glycosylphosphatidylinositol-anchored proteins are preferentially targeted to the basolateral surface in Fischer rat thyroid epithelial cells. J Cell Biol. 1993 Jun;121(5):1031–1039. doi: 10.1083/jcb.121.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo C., van't Hof W., van Meer G., Rodriguez-Boulan E. VIP21/caveolin, glycosphingolipid clusters and the sorting of glycosylphosphatidylinositol-anchored proteins in epithelial cells. EMBO J. 1994 Jan 1;13(1):42–53. doi: 10.1002/j.1460-2075.1994.tb06233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink M. M., Edison A. M., Wells G. B., Pinto W., Lester R. L. Characterization of a novel, potent, and specific inhibitor of serine palmitoyltransferase. J Biol Chem. 1992 Dec 15;267(35):25032–25038. [PubMed] [Google Scholar]
- van Meer G. Transport and sorting of membrane lipids. Curr Opin Cell Biol. 1993 Aug;5(4):661–673. doi: 10.1016/0955-0674(93)90137-f. [DOI] [PubMed] [Google Scholar]