Figure 2.
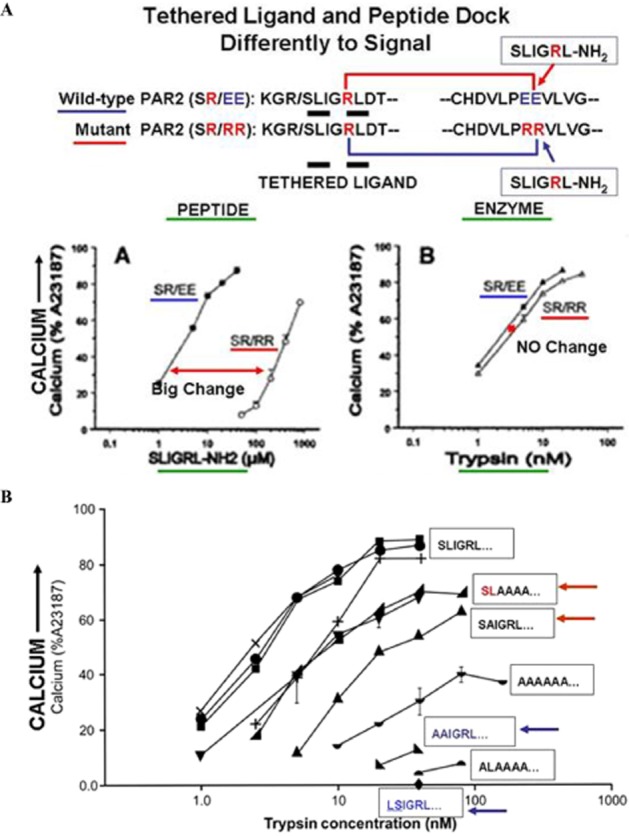
Differences in signalling by PAR2-activating peptide versus TL in a mutant PAR2 ‘SR/RR’ receptor (A, upper, panels A and B) and minimal TL sequence needed for calcium signalling (B, lower). (A, upper) The scheme at the top shows the wild-type receptor sequence (PAR2: SR/EE), with a TL sequence, ‘SLIGRL---) thought to interact with the ‘PEE’ sequence in extracellular loop-2. This wild-type receptor is compared with a mutant PAR2 having the same ‘TL’ (SLIGR---) but a mutated ‘PRR’ sequence in extracellular loop 2. The upper-left figure A shows calcium signalling stimulated by the PAR2-activating peptide, SLIGRL-NH2 in the wild-type (SR/EE), compared with the mutant (SR/RR) receptor, demonstrating a marked rightward shift in the concentration-effect curve for the mutant (SR/RR) versus the wild-type receptor (SR/EE). In contrast, the upper-right panel B shows there is no difference between the wild-type (SR/EE) versus mutant (SR/RR) receptor for activation by the trypsin-revealed TL. Thus, the receptor-docking sites for signalling by the synthetic PAR-activating peptide and the proteinase-unmasked TLs differ. Adapted from Al-Ani et al., 2002 with permission. (B, lower) A minimal PAR2-TL sequence (SL----) is required for calcium signalling. The proteinase-revealed ‘TL’ sequence of PAR2 was mutated so that trypsin cleavage unmasked different mutated ‘TL’ sequences with alanine substitutions (sequences shown in boxes) in the first six amino acids. When revealed, the mutated TL sequences, SLAAAA--- and SAIGRL--- were able to stimulate calcium signalling (red arrows, upper curves), whereas the sequences, AAIGRL--- and LSIGRL--- did not (blue arrows, bottom). Adapted from Al-Ani et al., 2004, with permission.
