Figure 5.
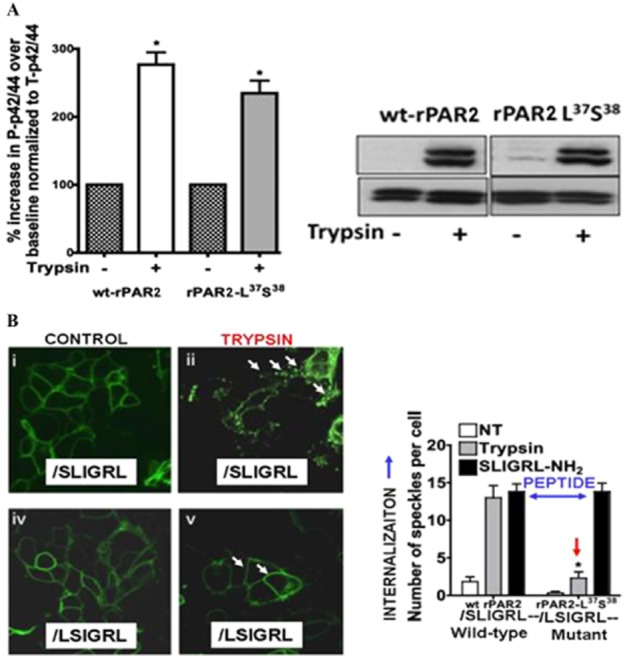
Biased MAPKinase signalling by a mutated rat PAR2-TL (A) without receptor internalization (B). (A, upper) Activation of PAR2 MAPKinase signalling by the trypsin-exposed mutated TL, //L37S38IGRL--- (rPAR2-L37S38). PAR2 was mutated so that trypsin cleavage (shown by //) at the R36//L37SIGRL--- sequence unmasks the TL sequence, L37S38IGRL--- (rPAR2-L37S38), which activates MAPKinase to the same level as for trypsin-activated wild-type receptor [A, histograms (left) and Western blot (right)]. However, the trypsin-unmasked mutant TL (rPAR2-L37S38) does not activate calcium signalling (Figure 2B, lower panel, blue arrow at the bottom, X-axis). (B) However, activation of the mutant PAR2 (rPAR2-L37S38) by the trypsin-exposed TL, //LSIGRL--- does not trigger receptor internalization (lower-right image, Figure 5B; red arrow, right histograms). In contrast, peptide-driven receptor activation causes internalization of both the wild-type and mutant rPAR2-L37S38 receptors (blue two-way arrow, right-hand histograms, Figure 5B). Adapted from Ramachandran et al., 2009.
