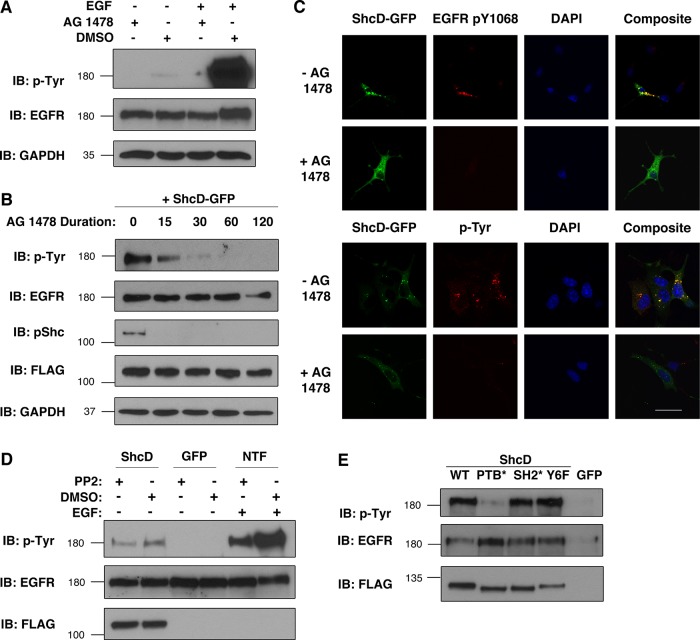
FIGURE 4:
ShcD-induced EGFR phosphorylation requires the EGFR kinase and the ShcD PTB domain. (A) The capacity of 1 μM AG 1478 (Tyrphostin) vs. DMSO (vehicle) to prevent EGFR phosphorylation was verified using untransfected COS-1 cells in the presence and absence of 50 ng/ml EGF (10 min). (B) Subsequently, ShcD-overexpressing cells were serum starved and exposed to AG 1478 for varying amounts of time (in minutes) before lysis and immunoblotting. (C) The interplay between ShcD, the intrinsic EGFR kinase, and the cellular tyrosine phosphorylation profile was further analyzed via confocal microscopy of cells incubated with AG 1478 for 30–60 min and probed with anti-EGFR-pY1068 and anti-pTyr antibody. Scale bar, 40 μm. (D) Similarly, the SFK inhibitor PP2 (10 μM) was evaluated for its ability to counteract both ligand-induced (10 ng/ml, 10 min) and ShcD-induced EGFR phosphorylation. NTF, nontransfected cells. (E) To elucidate the molecular region of ShcD responsible for promoting EGFR phosphorylation, point-mutated variants of the protein containing disrupted PTB or SH2 domains (PTB*, SH2*) or the phosphorylation-defective tyrosine-to-phenylalanine substitutions (Y6F) were overexpressed in cells. Lysates from serum-deprived samples were immunoblotted for pTyr. IB, immunoblot.